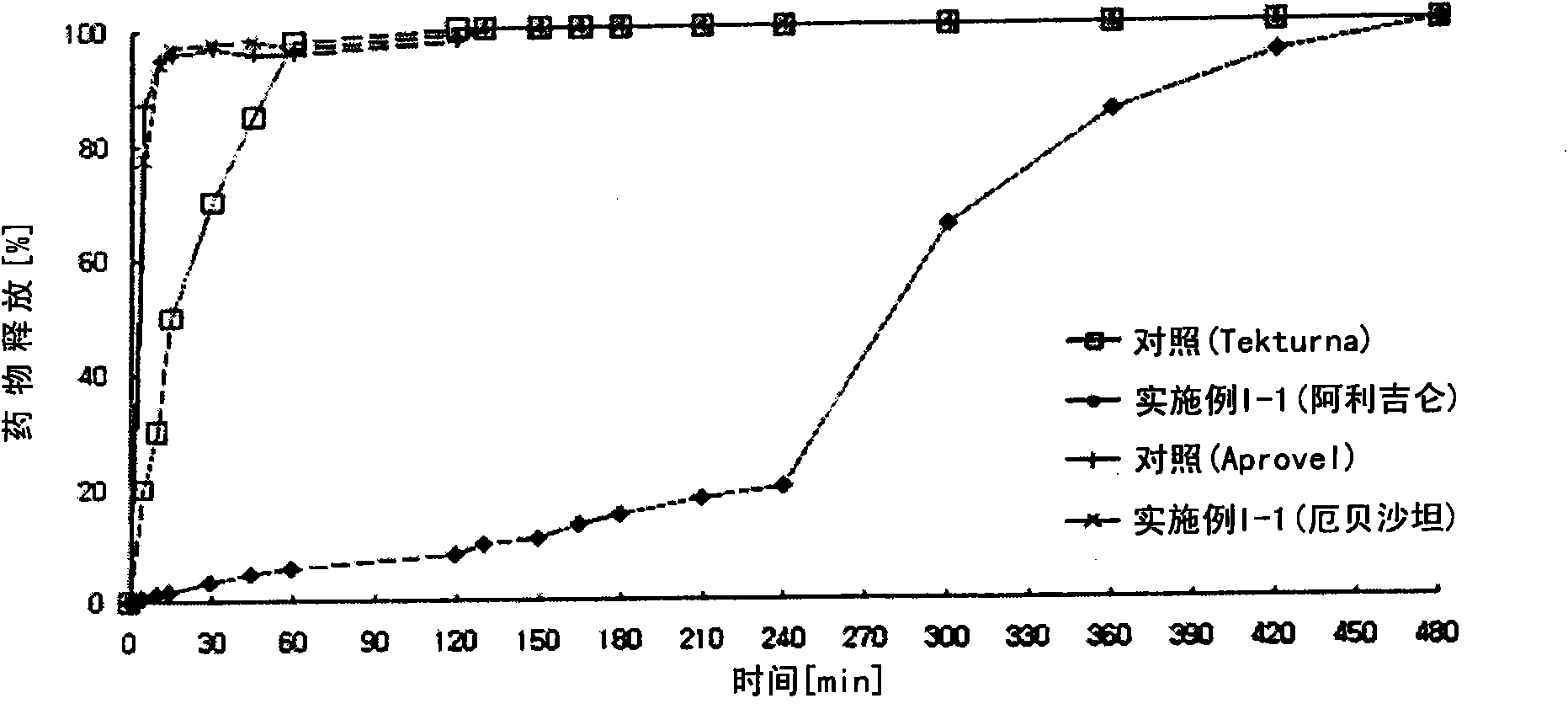
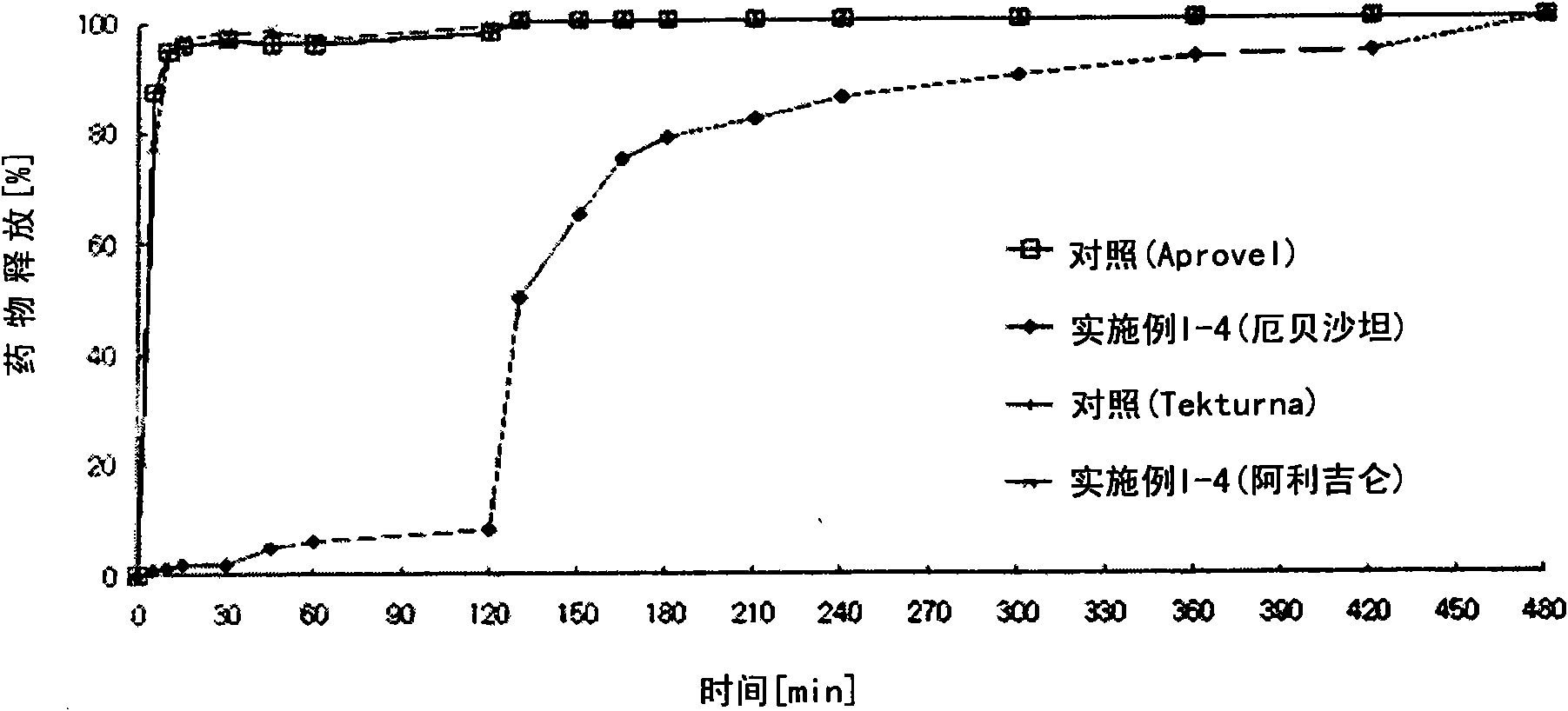
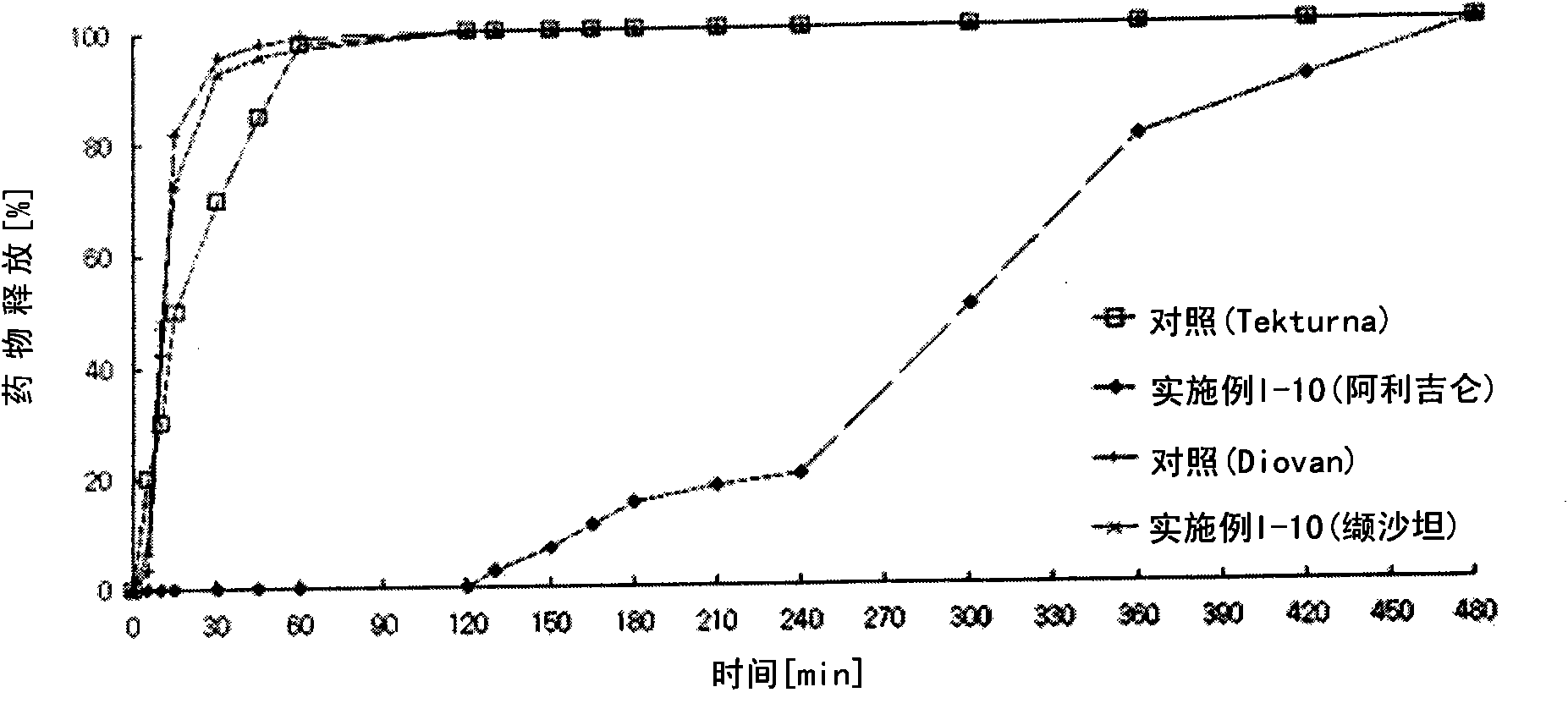
Pharmaceutical formulation
A technology of pharmaceutical preparations and renin inhibitors, applied in the field of pharmaceutical preparations, can solve the problems of not providing the cumulative effect of combined preparations and the decline in the effect of aliskiren, and achieve the effect of improving the problem of simultaneous administration and effective curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
[0141]
Embodiment I-1
[0142] Embodiment I-1: the preparation of biphasic matrix tablet
[0143] According to the ingredient composition and content shown in Table 1 below, it was prepared through the following steps.
[0144] 1) Preparation of sustained-release compartments containing aliskiren
[0145] Aliskiren hemifumarate, microcrystalline cellulose, crospovidone, and sodium chloride were sieved through a No. 35 sieve and mixed in a high speed mixer for 5 minutes to prepare a mixture. Meanwhile, polyvinylpyrrolidone was dissolved in purified water to prepare a binding solution (10 w / w%). The binding solution and the mixture of the main ingredients are placed in a high-speed mixer, and then kneaded. After completing the kneading process, the kneaded material was granulated using a shaker with a No. 20 sieve, and the granules were dried in a hot water drier at 60°C. Meanwhile, cellulose acetate (acetyl 32%), cellulose acetate (acetyl 39.8%), and hydroxypropylmethylcellulose were dissolved ...
Embodiment I-2
[0150] Embodiment 1-2: the preparation of biphasic matrix tablet
[0151] According to the ingredient composition and content shown in Table 1 below, it was prepared through the following steps.
[0152] 1) Preparation of sustained-release compartments containing aliskiren
[0153] Aliskiren hemifumarate and microcrystalline cellulose were sieved through a No. 35 sieve and mixed in a high-speed mixer for 5 minutes to prepare a mixture. Meanwhile, polyvinylpyrrolidone was dissolved in purified water to prepare a binding solution (10 w / w%). The mixture of the binding solution and the main ingredients is placed in a high-speed mixer and then kneaded. After completing the kneading process, the kneaded material was granulated using a shaker with a No. 20 sieve, and the granules were dried in a hot water drier at 60°C. Then, a mixed solution (10% w / w) of hydroxypropyl methylcellulose and hydroxypropyl methylcellulose phthalate in a 1:1 mixture of ethanol and methylene chlorid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com