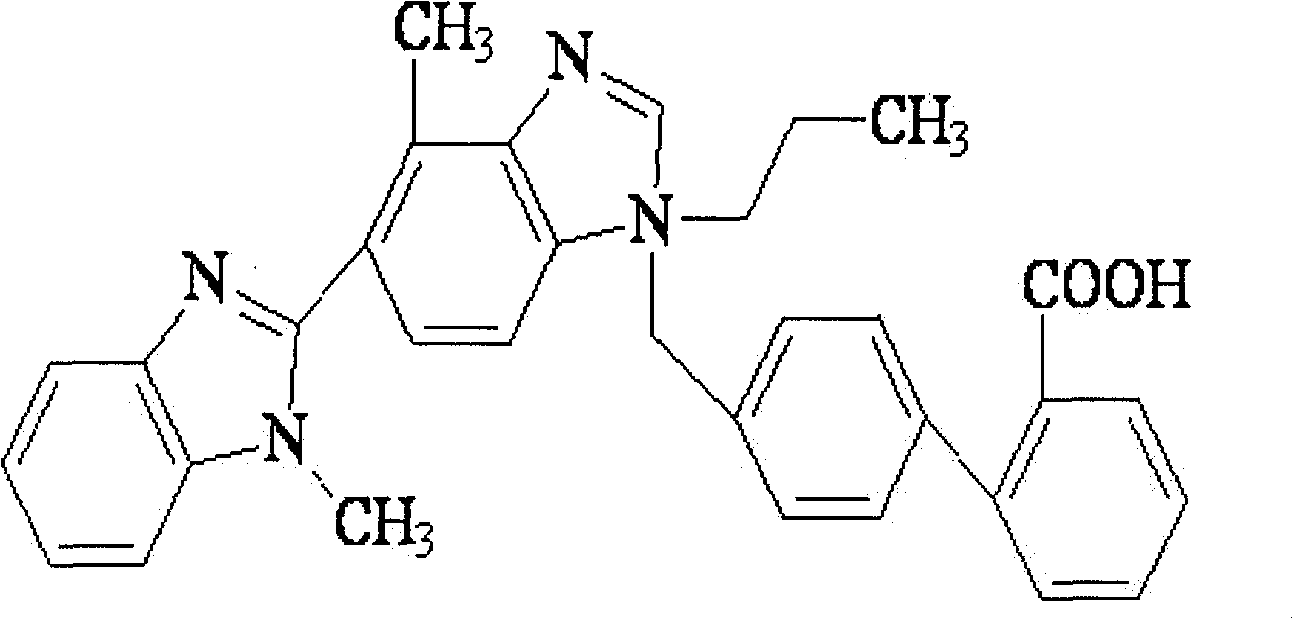
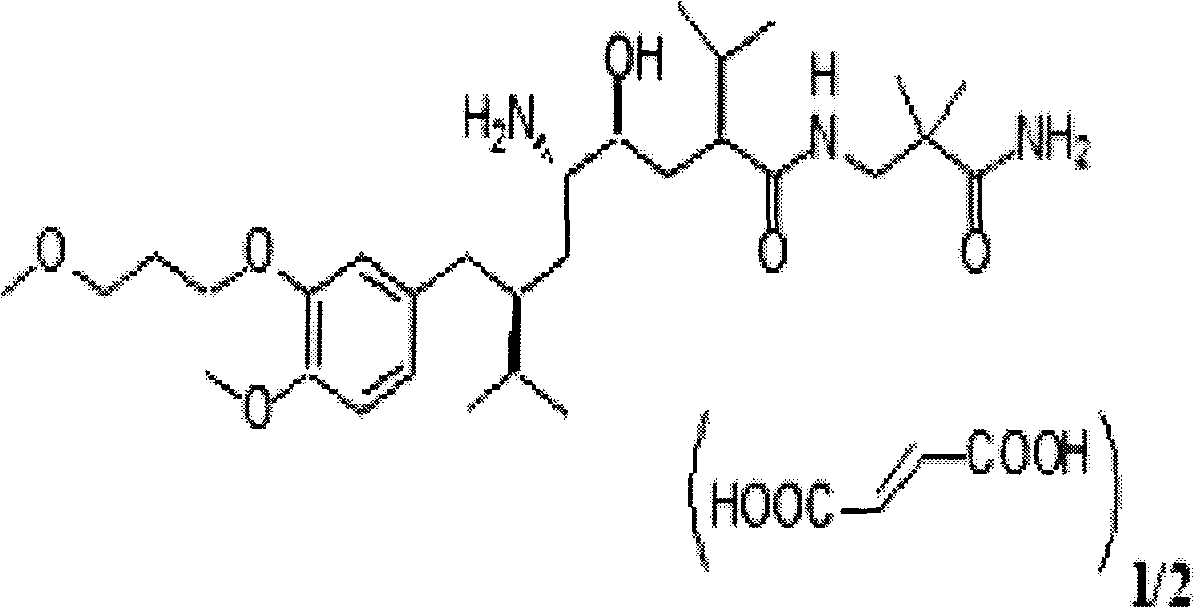
Combined medicament containing telmisartan and aliskiren and preparation method thereof
A technology of telmisartan and medicine, applied in the field of combined medicine containing telmisartan and Aliskiren and its preparation, to achieve the effect of maintaining stability and avoiding interaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
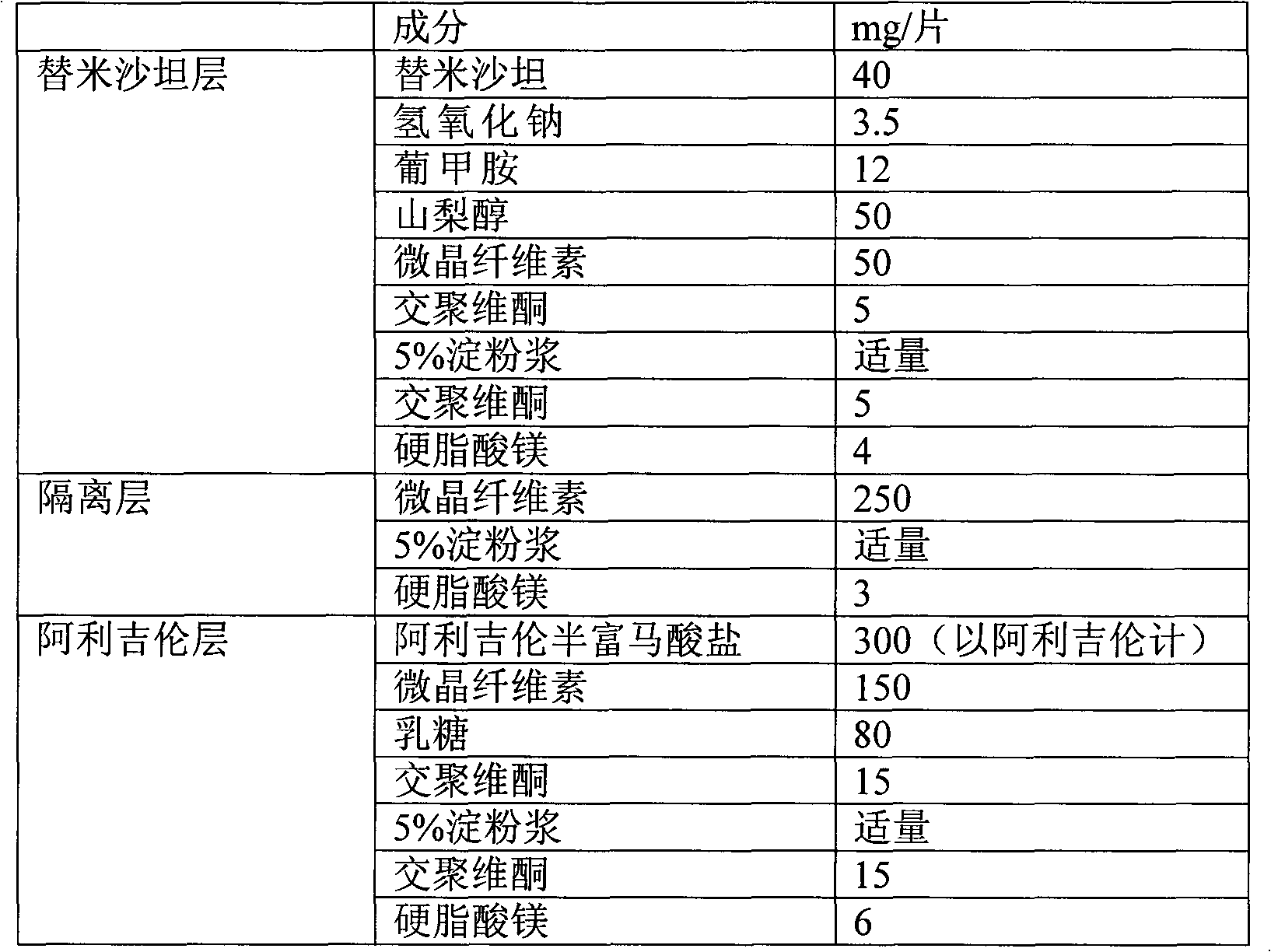
[0037] The preparation of embodiment 1 medicine of the present invention
[0038] Prescription composition:
[0039]
[0040] operate:
[0041] 1. Dissolve the prescribed amount of telmisartan with the prescribed amount of sodium hydroxide ethanol solution, then spray dry, and pass the dry powder through an 80-mesh sieve. The sifted powder, meglumine, mannitol, microcrystalline cellulose and internally added crospovidone were mixed uniformly in equal increments. Wet granulation with an appropriate amount of 5% starch slurry, drying at 50°C, sizing the granules with a 20-mesh sieve, adding crospovidone and magnesium stearate and mixing to obtain telmisartan layer granules.
[0042] 2. Mix the prescribed amount of Aliskiren with the prescribed amount of microcrystalline cellulose, lactose, and internally added crospovidone in equal increments, and wet granulate with an appropriate amount of 5% starch slurry; dry at 50°C , granulated with a 20-mesh sieve, added crospovidone...
Embodiment 2
[0045] The preparation of embodiment 2 medicines of the present invention
[0046] Prescription composition:
[0047]
[0048] operate:
[0049] 1. Dissolve the prescribed amount of telmisartan with the prescribed amount of sodium hydroxide ethanol solution, then spray dry, pass the dry powder through an 80-mesh sieve, and sieve the powder, meglumine, mannitol, and microcrystalline cellulose Mix with the internally added crospovidone in an equal amount, wet granulate with an appropriate amount of 5% starch slurry, and dry at 50°C. Use a 20-mesh sieve to sieve the granules, add crospovidone and magnesium stearate and mix well to obtain telmisartan layer granules.
[0050] 2. Mix the prescribed amount of Aliskiren with the prescribed amount of microcrystalline cellulose, lactose and internally added crospovidone in an equal and progressive manner, wet granulate with an appropriate amount of 5% starch slurry, and dry at 50°C , granulated with a 20-mesh sieve, added crospovi...
Embodiment 3
[0053] Embodiment 3 preparation of medicine of the present invention
[0054] Prescription composition:
[0055]
[0056] operate:
[0057] 1. Dissolve the prescribed amount of telmisartan with the prescribed amount of sodium hydroxide ethanol solution, then spray dry, pass the dry powder through an 80-mesh sieve, and sieve the powder, meglumine, mannitol, and microcrystalline cellulose Mix with the internally added crospovidone in an equal amount, wet granulate with an appropriate amount of 5% starch slurry, and dry at 50°C. Use a 20-mesh sieve to sieve the granules, add crospovidone and magnesium stearate and mix well to obtain telmisartan layer granules.
[0058] 2. Mix the prescribed amount of Aliskiren with the prescribed amount of microcrystalline cellulose, lactose and internally added crospovidone in an equal and progressive manner, wet granulate with an appropriate amount of 5% starch slurry, and dry at 50°C , granulated with a 20-mesh sieve, added crospovidone ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com