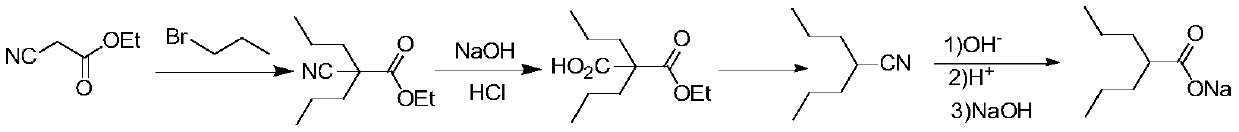
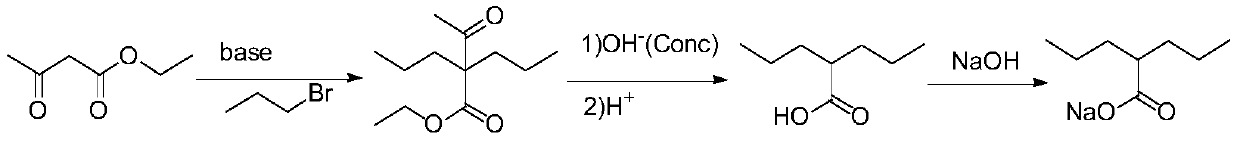
Preparation method of sodium valproate
A technology of sodium valproate and halopropane, applied in the field of pharmaceutical synthesis, can solve the problems of low utilization rate of equipment, long synthesis route, low total reaction yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
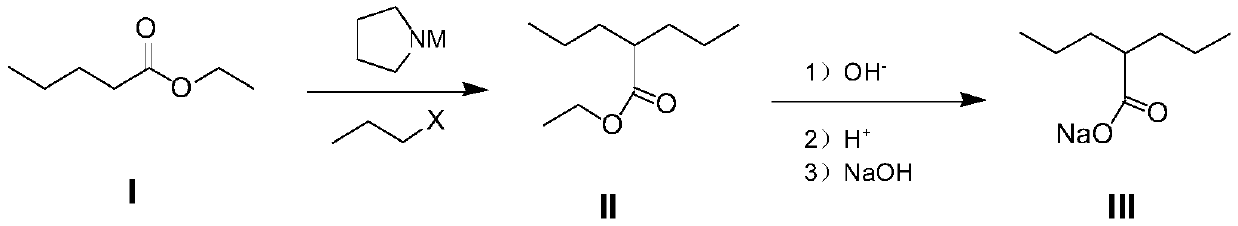
[0033] Embodiment 1, the method for synthesizing sodium valproate of the present invention
[0034] (1) Preparation of compound II
[0035] 2kg of ethyl valerate (compound I) was dissolved in 20kg of methyl tert-butyl ether, under the protection of nitrogen, the temperature was lowered to -25°C, and then 6.77kg (8.46L, density 0.8kg / L) 2mol / L was added dropwise The methyl tert-butyl ether solution of the pyrrole lithium reagent was added dropwise. After stirring for 2 hours, the temperature of the reaction system was controlled at -30°C, and 2.08 kg of bromopropane was added dropwise. The 10% ammonium chloride solution of 5kg terminated the reaction, and after standing still for 20 minutes, the aqueous phase was separated; the organic phase was washed once with 3kg of saturated saline; the aqueous phase was separated, and the organic solvent was concentrated under reduced pressure to obtain compound II .
[0036] (2) Preparation of compound III
[0037] Dissolve the compoun...
Embodiment 2
[0038] Embodiment 2, the method for synthesizing sodium valproate of the present invention
[0039] (1) Preparation of compound II
[0040]3kg of ethyl valerate (compound I) was dissolved in 24kg of methyl tetrahydrofuran, under the protection of nitrogen, the temperature was lowered to -40°C, and then 13.85kg (17.31L, density 0.8kg / L) of 2mol / L lithium pyrrole was added dropwise The methyl tert-butyl ether solution of the reagent was added dropwise, and after stirring for 3 hours, the temperature of the reaction system was controlled at -30°C, and 3.97 kg of bromopropane was added dropwise. After the reaction of the raw materials was completed, 8 kg of 10 % ammonium chloride solution to terminate the reaction, and after standing still for 30 minutes, the aqueous phase was separated; the organic phase was washed once with 5 kg of saturated brine; the aqueous phase was separated, and the organic solvent was concentrated under reduced pressure to obtain compound II.
[0041] (2...
Embodiment 3
[0043] Embodiment 3, the method for synthesizing sodium valproate of the present invention
[0044] (1) Preparation of compound II
[0045] 5kg of ethyl valerate (compound I) was dissolved in 45kg of tetrahydrofuran, under the protection of nitrogen, the temperature was lowered to -35°C, and then 20kg (25.0L, density 0.8kg / L) of 2mol / L pyrrole lithium reagent was added dropwise. Base tert-butyl ether solution, after the dropwise addition, after stirring for 3 hours, the temperature of the reaction system was controlled at -15°C, and 6.15kg of bromopropane was added dropwise. Ammonium chloride solution was used to terminate the reaction, and after standing still for 30 minutes, the aqueous phase was separated; the organic phase was washed once with 8.2 kg of saturated brine; the aqueous phase was separated, and the organic solvent was concentrated under reduced pressure to obtain compound II.
[0046] (2) Preparation of compound III
[0047] Dissolve compound II obtained in t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com