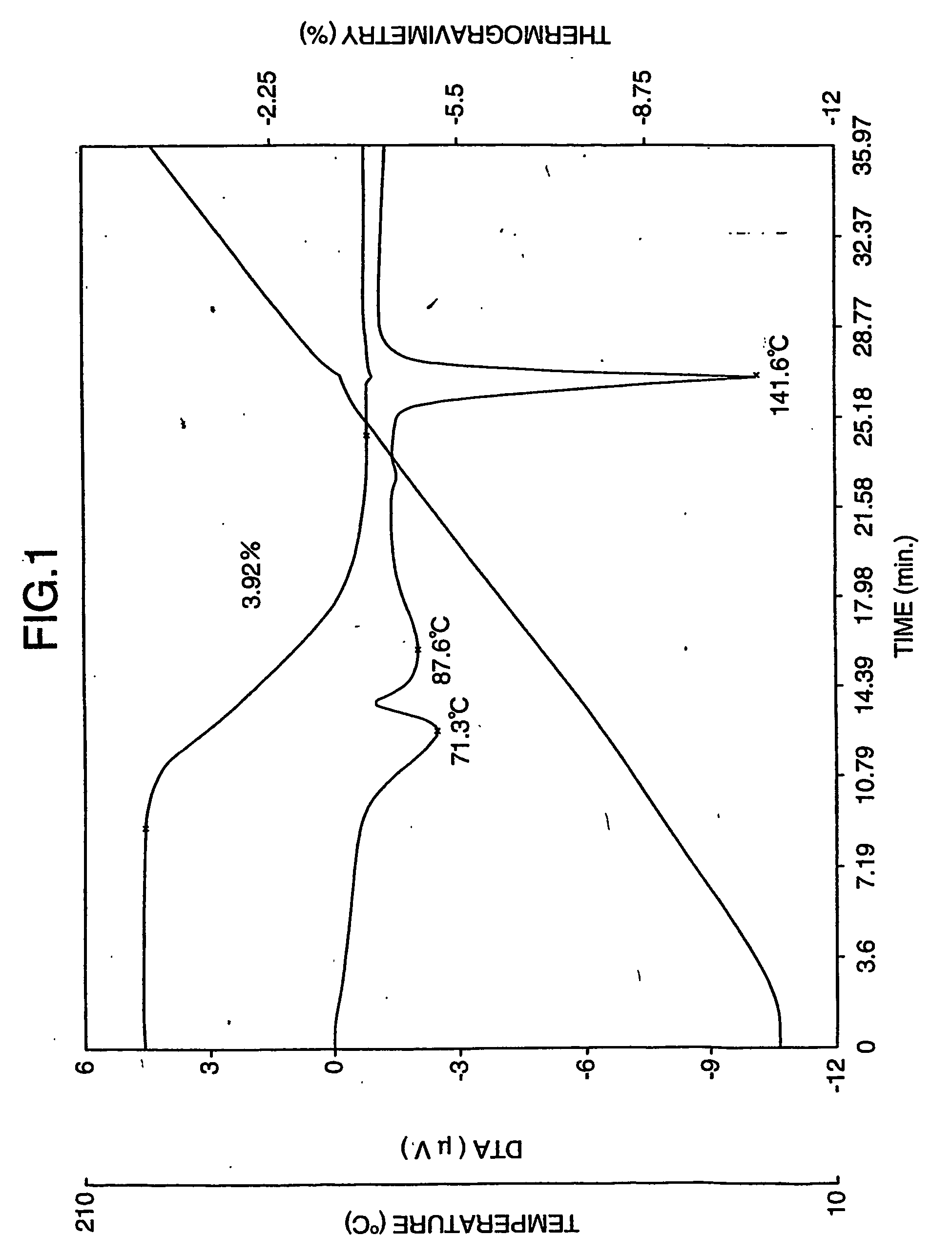
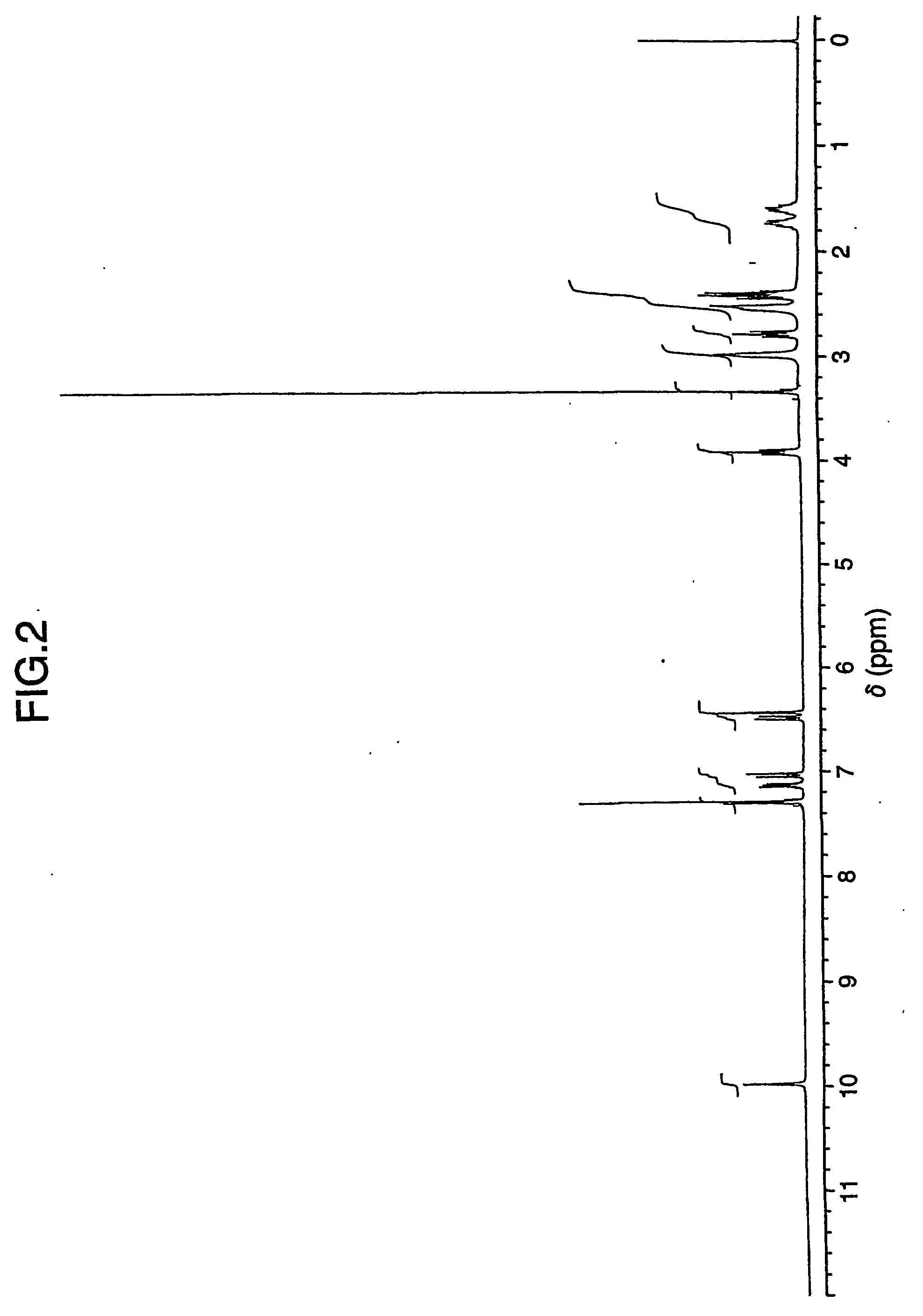
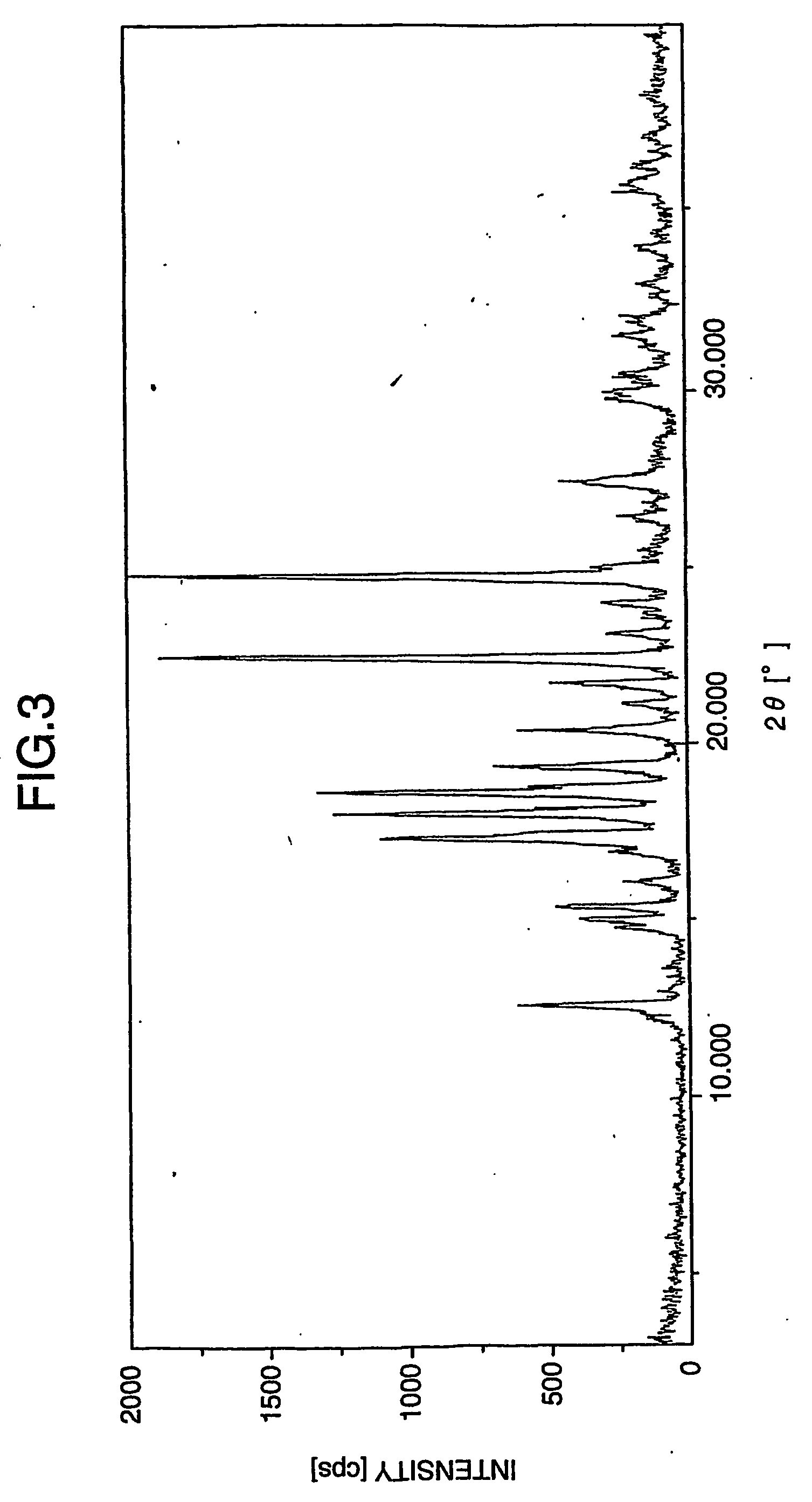
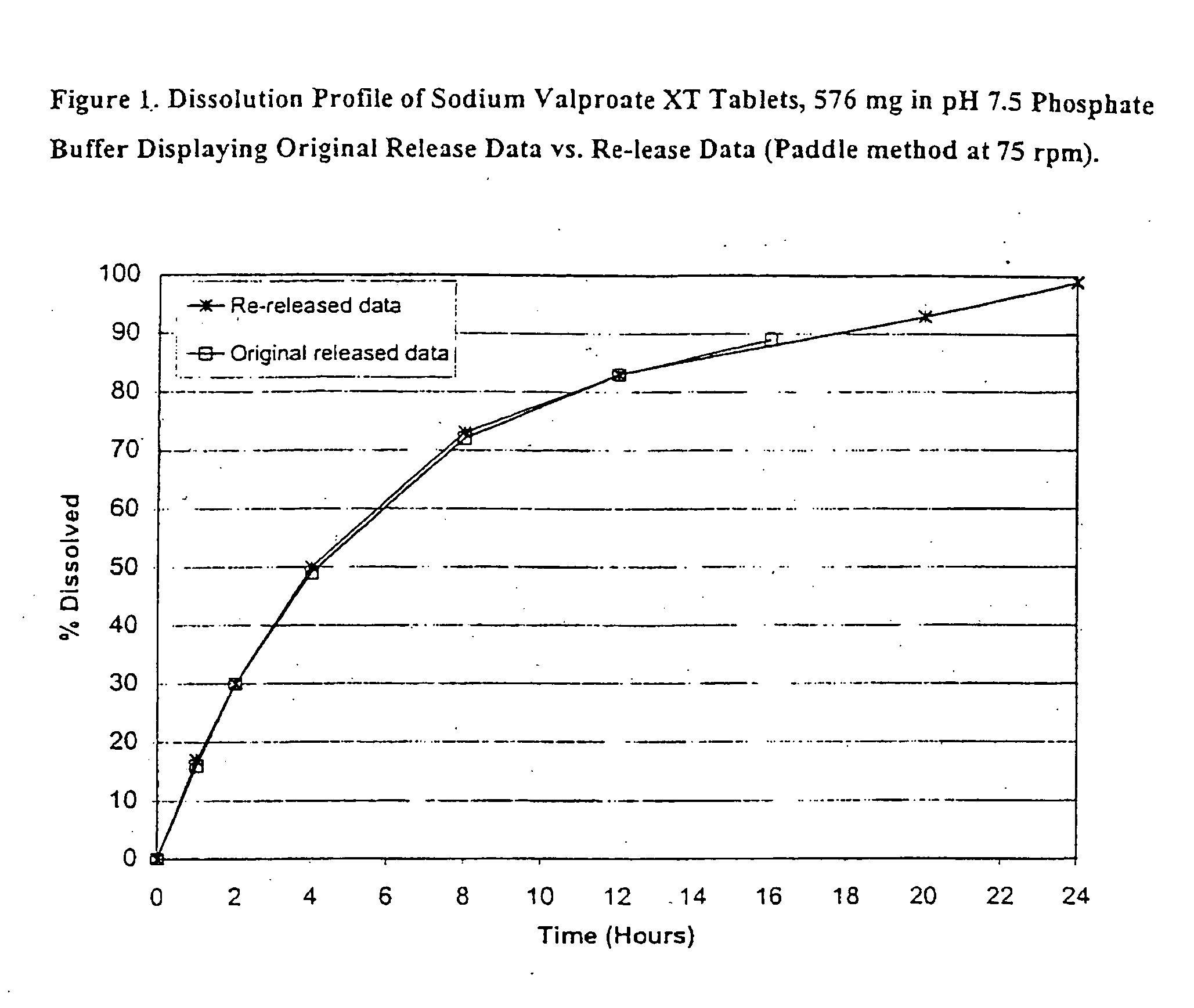
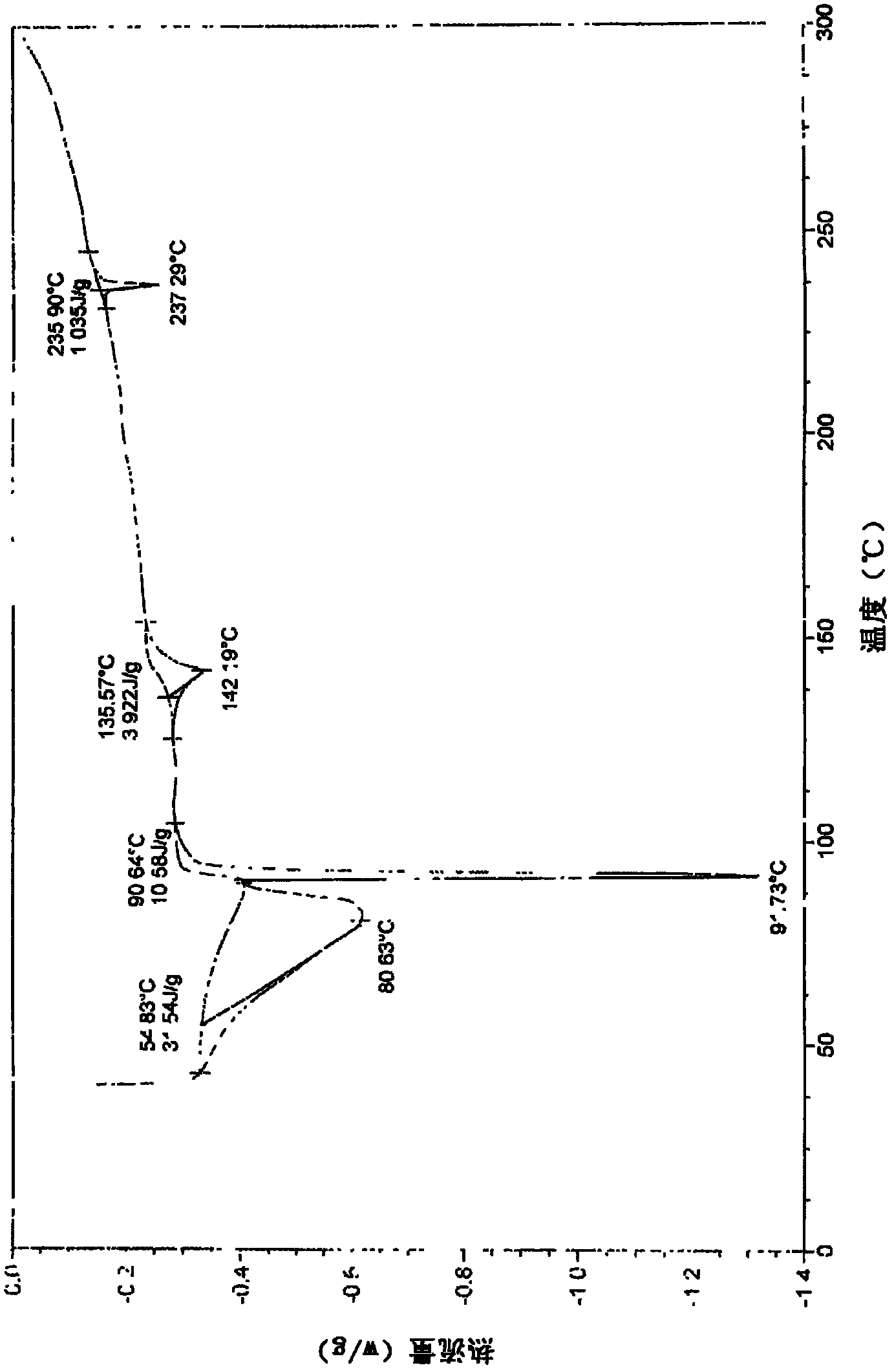
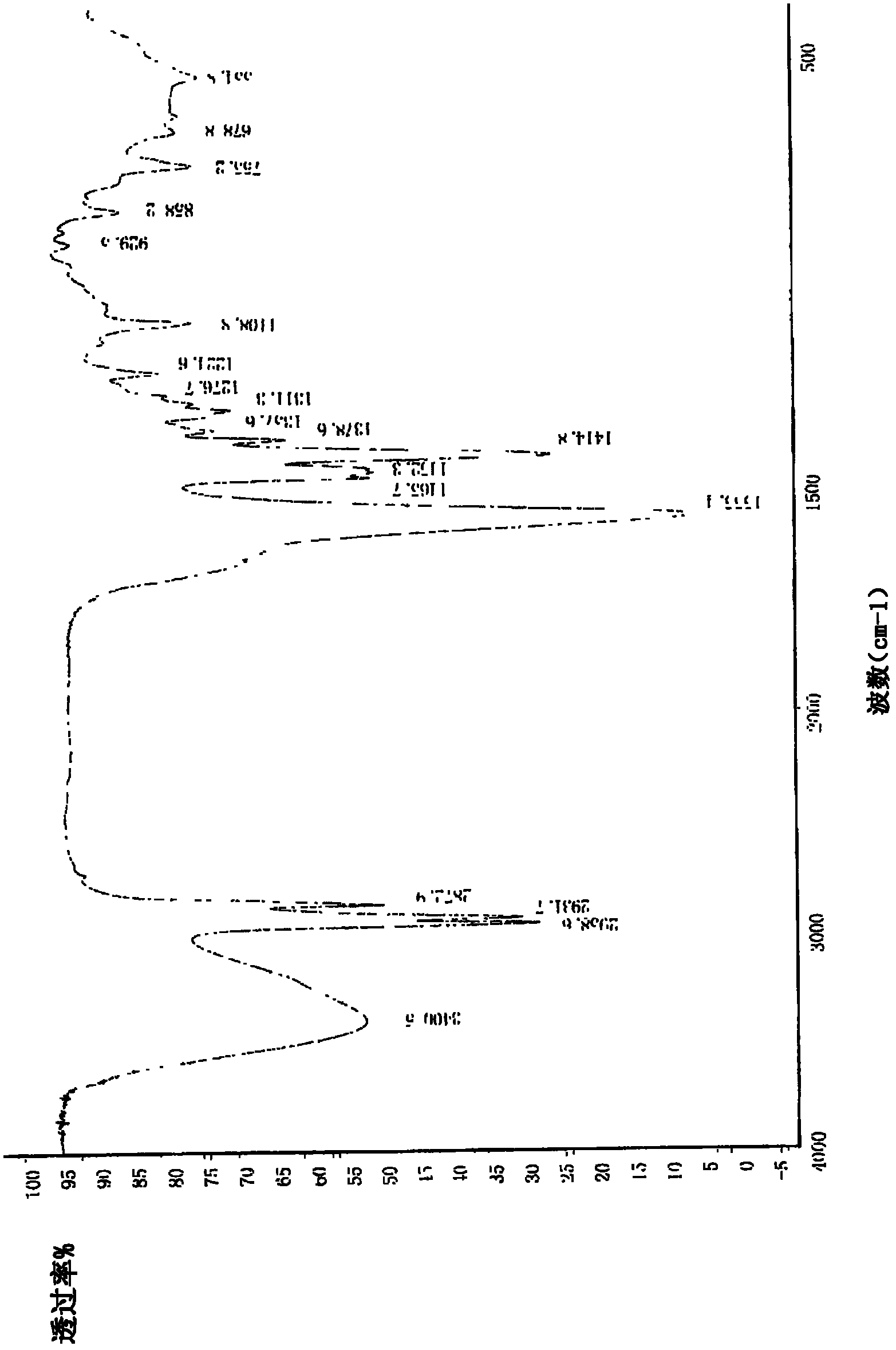
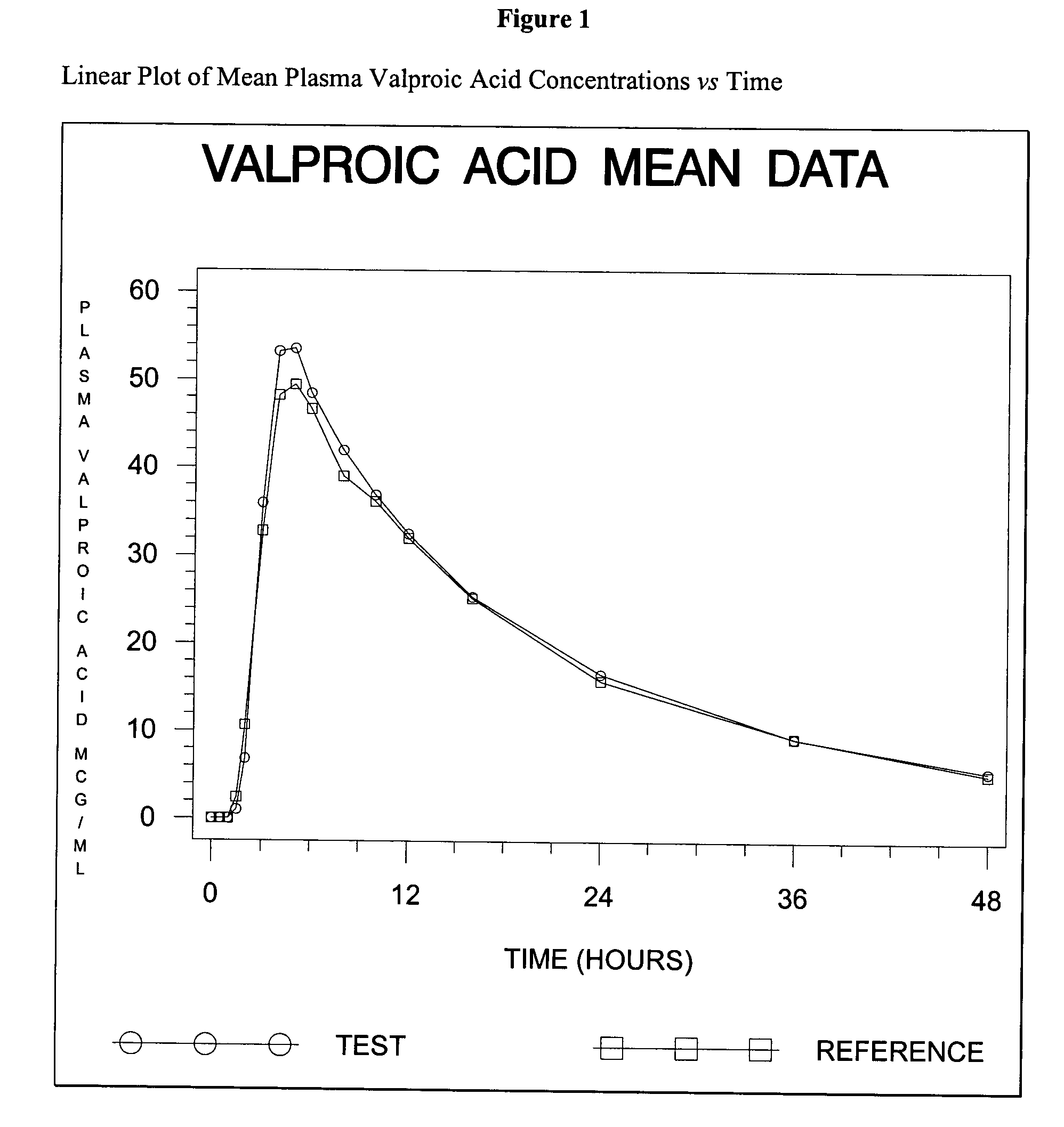
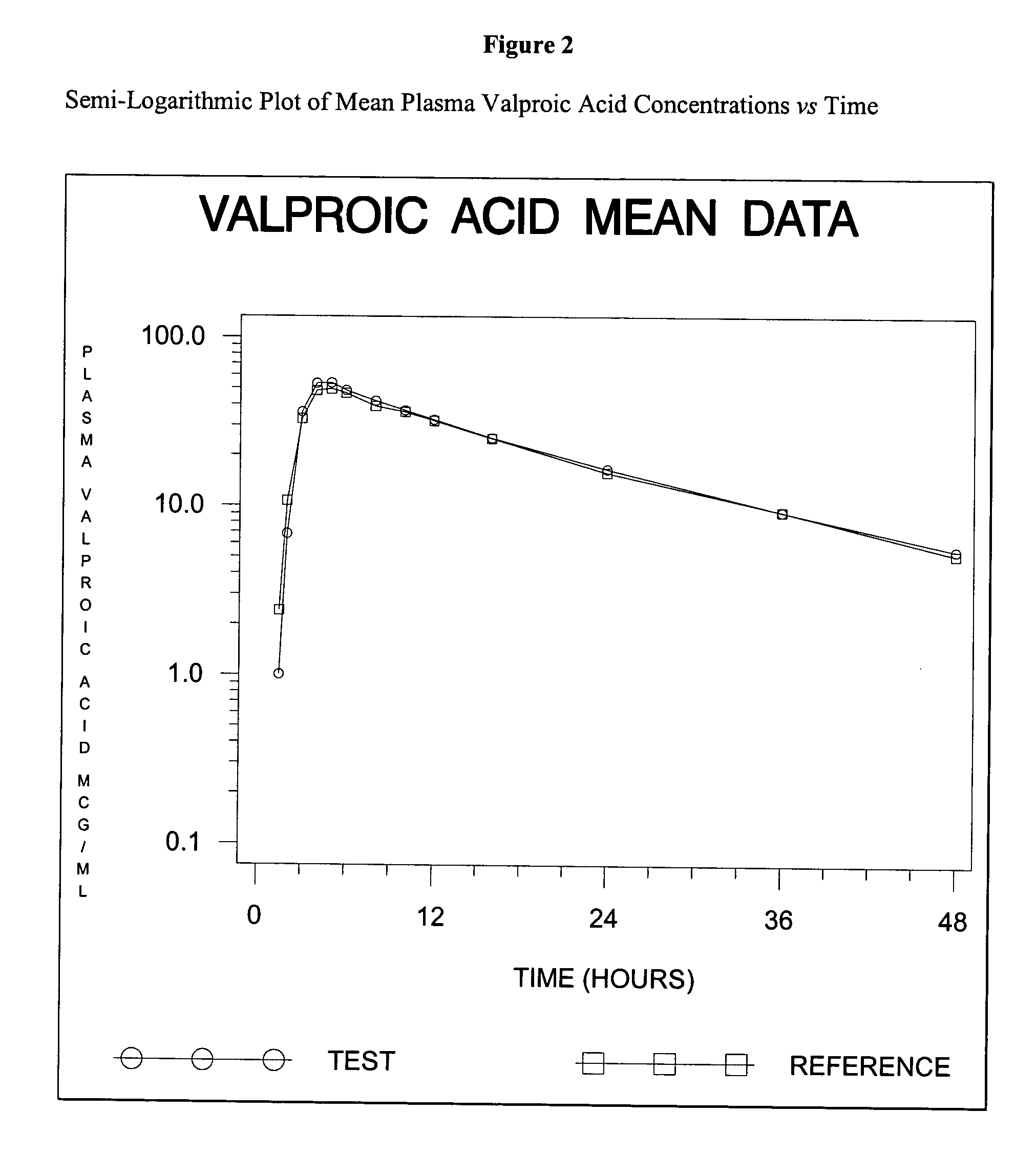
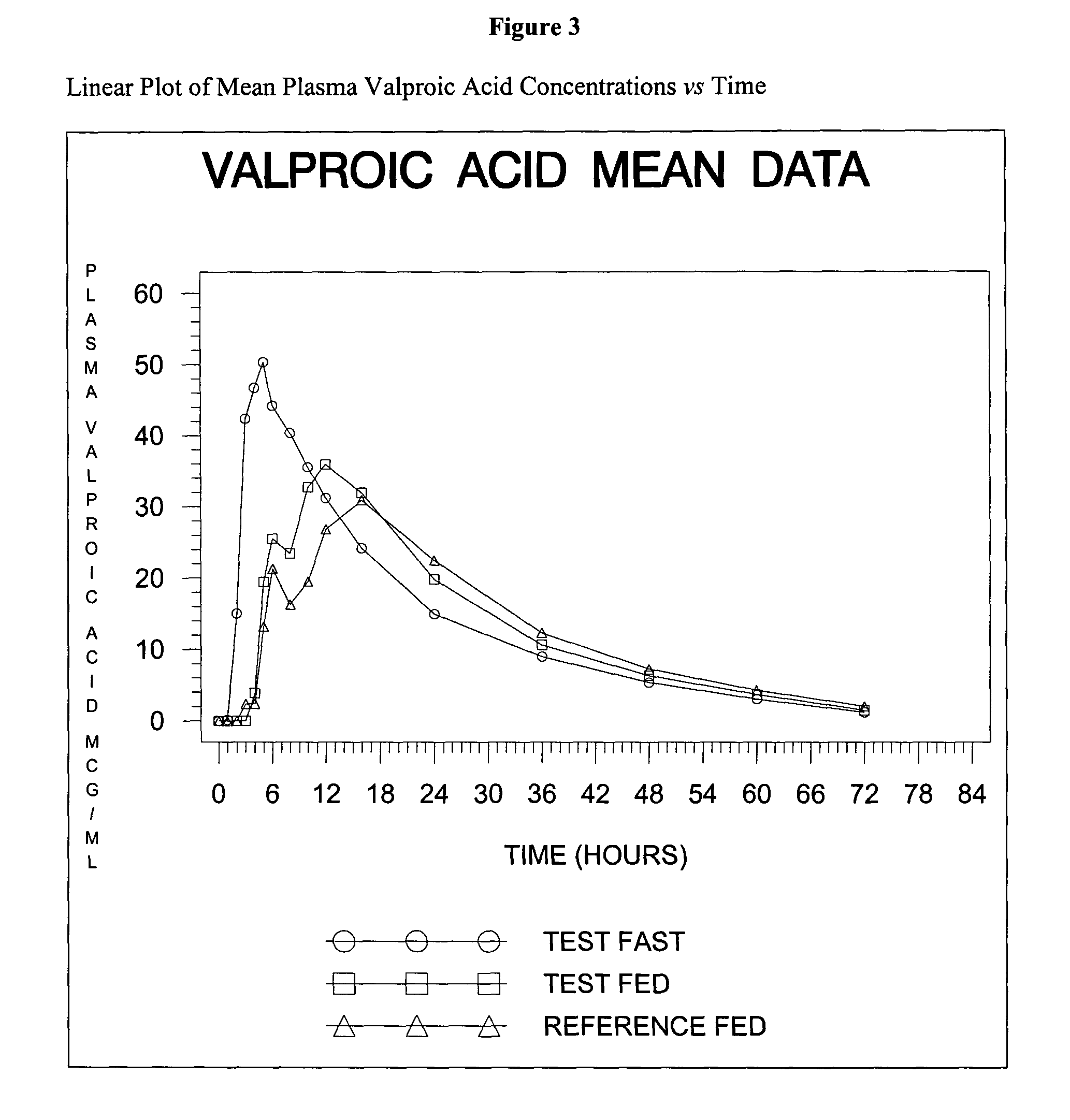
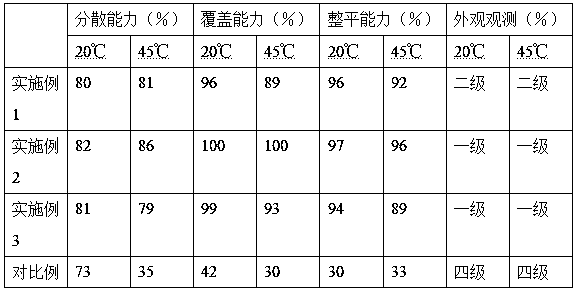
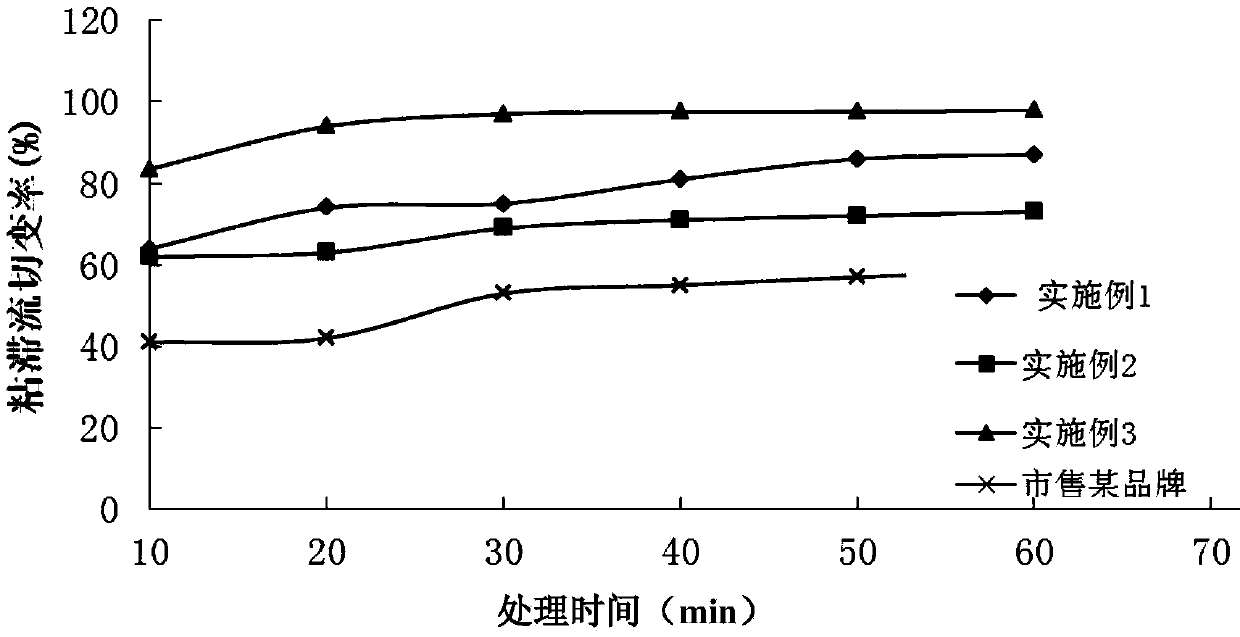
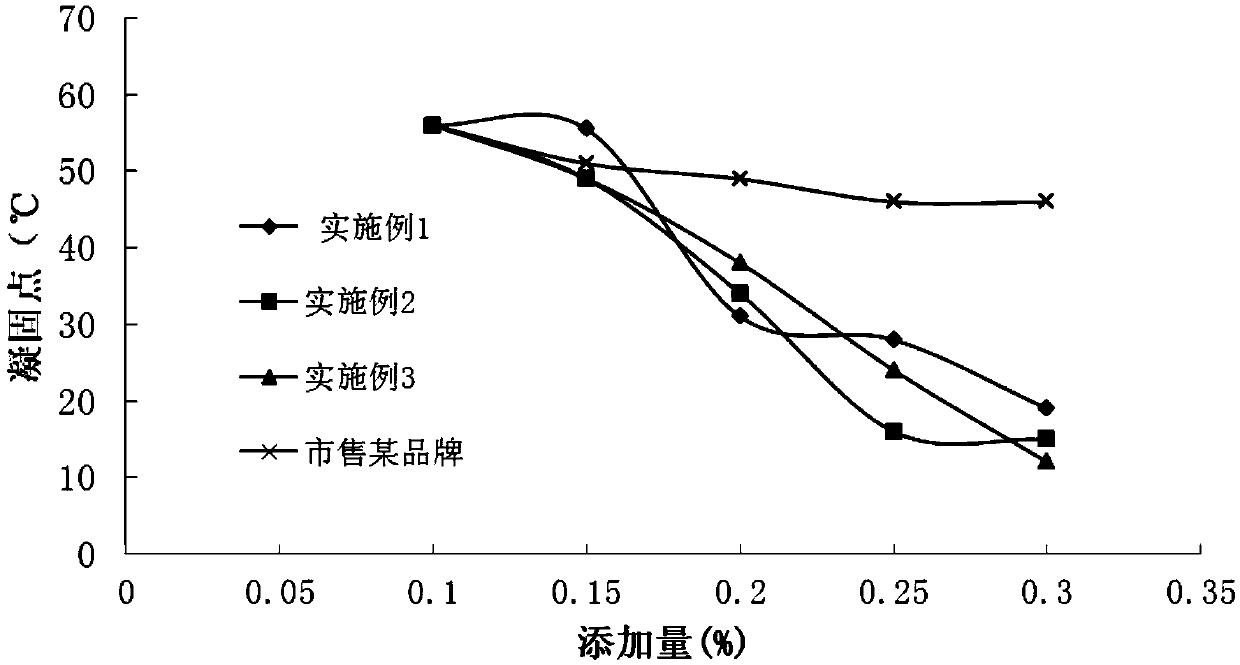
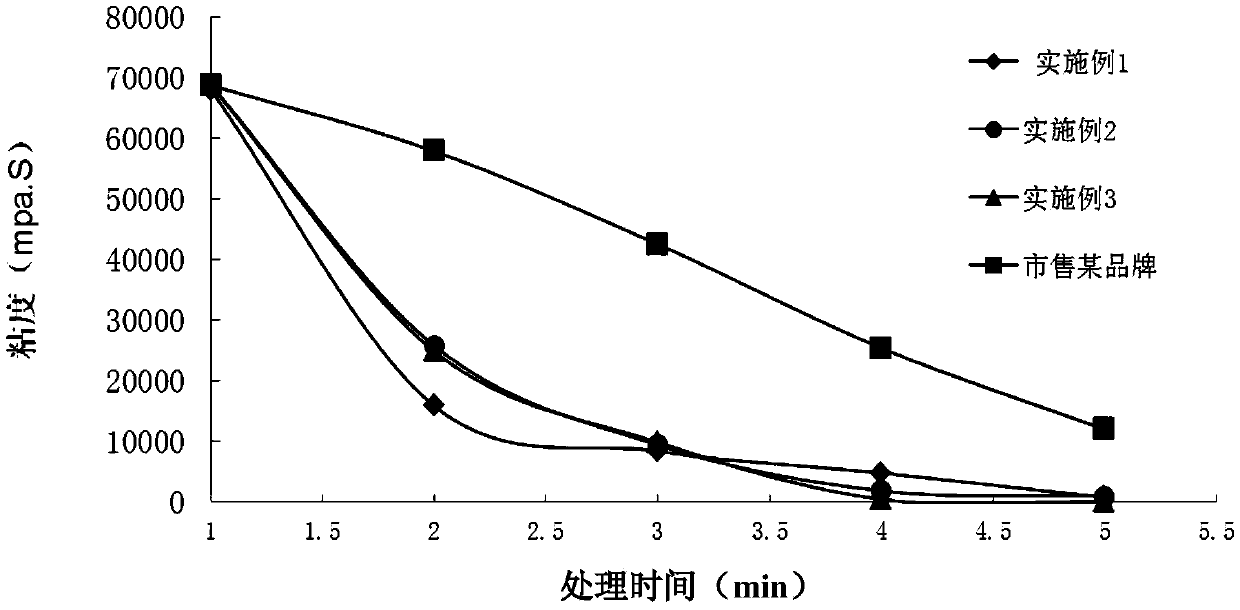
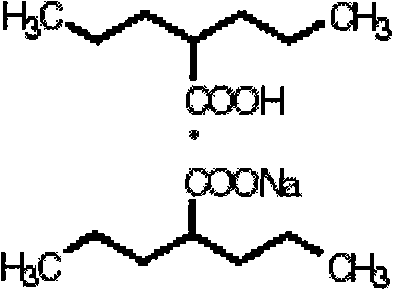
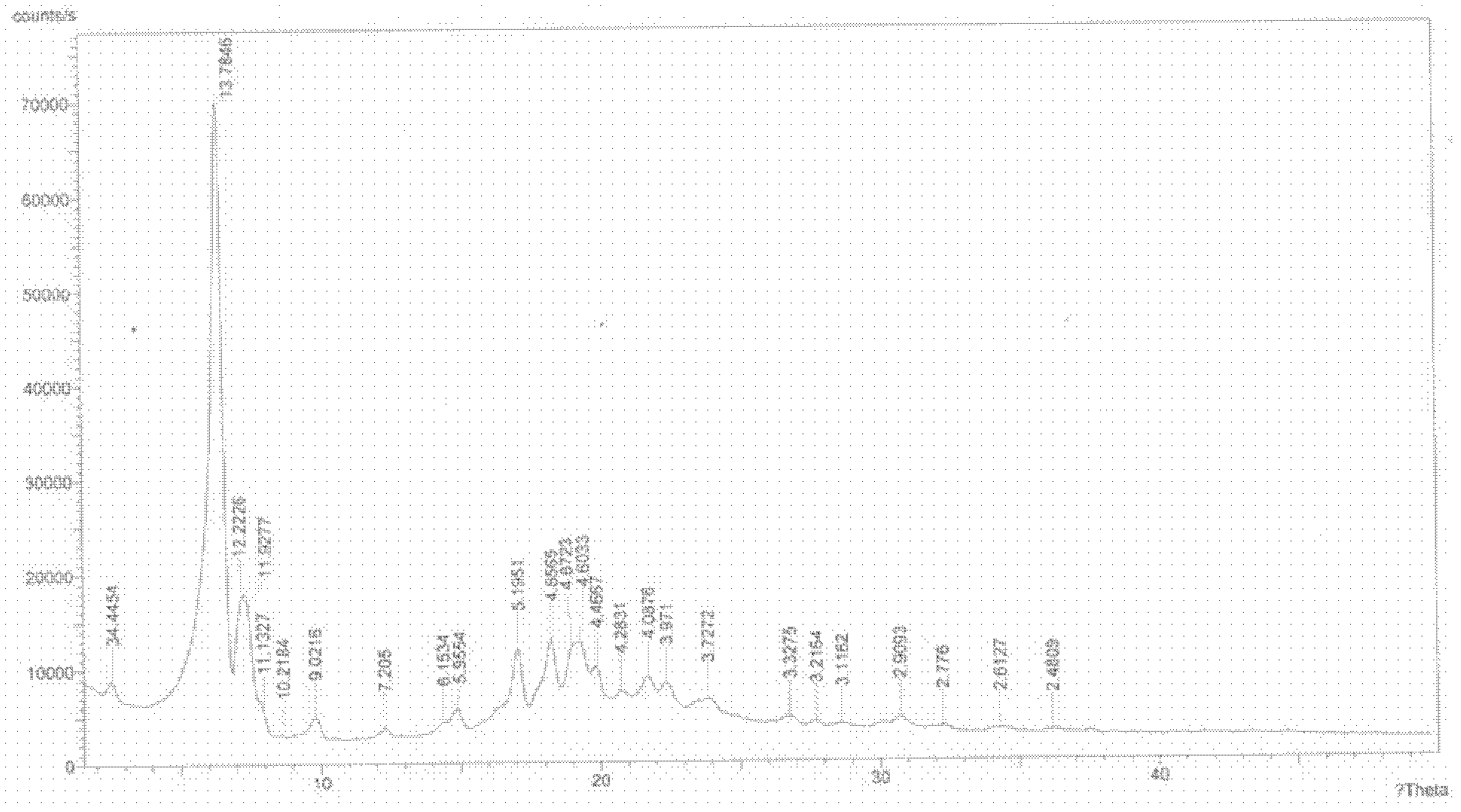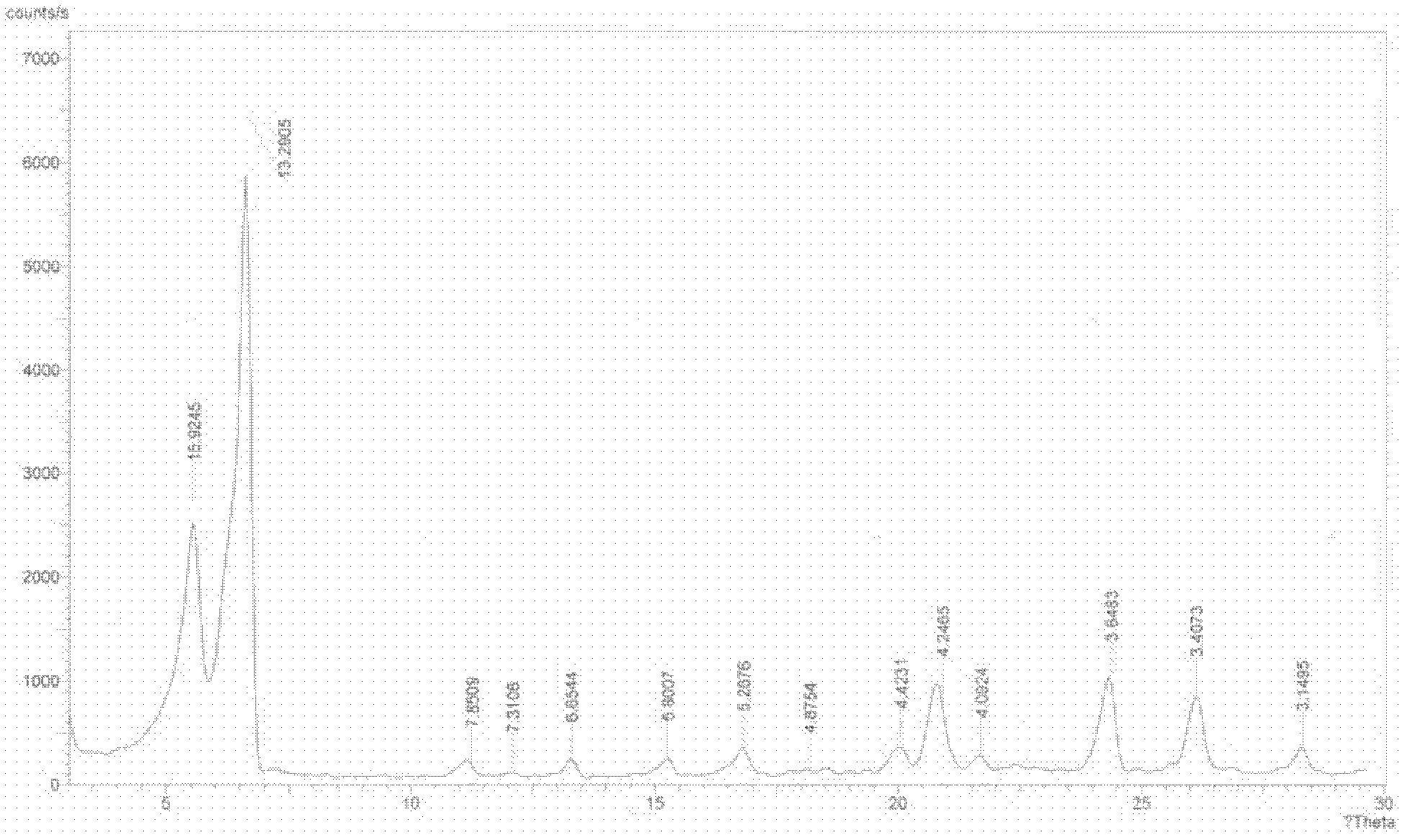Patents
Literature
103 results about "Valproate Sodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
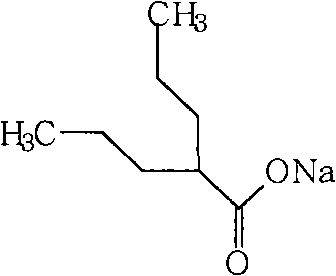
Sodium valproate or valproate sodium is the sodium salt of valproic acid and is an anticonvulsant used in the treatment of epilepsy, anorexia nervosa, panic attack, anxiety disorder, posttraumatic stress disorder, migraine and bipolar disorder, as well as other psychiatric conditions requiring the administration of a mood stabiliser. Sodium valproate can be used to control acute episodes of mania and acute stress reaction. Side effects can include tiredness, tremors, nausea, vomiting and sedation. The intravenous formulations are used when oral administration is not possible. In pregnancy, valproate has the highest risk of birth defects of any of the commonly used antiepilepsy drugs. However, some epilepsy can only be controlled by valproate, and seizures also pose grave risk to mother and child. Some of the common adverse effects include tiredness, tremor, sedation and gastrointestinal disturbances. In addition, about 10% of the users experience reversible hair loss.
Carbostyril derivatives and mood stabilizers for treating mood disorders
The pharmaceutical composition of the present invention comprises a carbostyril derivative which is a dopamine-sero-tonin system stabilizer and a mood stabilizer in a pharmaceutically acceptable carrier. The carbostyril derivative may be aripiprazole or a metabolite thereof. The mood stabilizer may include but is not limited to lithium, valproic acid, divalproex sodium, carbamaza-pine, oxcarbamazapine, zonisamide, lamotragine, topiramate, gabapentin, levetiracetam or clonazepam. These compositions are used to treat patients with mood disorders, particularly bipolar disorder with or without psychotic features, mania or mixed episodes. Methods are provided for separate administration of a carbostyril derivative and a mood stabilizer to a patient with a mood disorder.
Owner:OTSUKA PHARM CO LTD
Divalproex sodium tablets
Owner:ANDRX LABS
Divalproex sodium tablets
A process for preparing divalproex sodium tablets is provided. The process comprises preparing a neutralized divalproex sodium solution by combining divalproex sodium, having a sodium valproate and a valproic acid moiety, with an aqueous solvent and a base, e.g., sodium hydroxide, the base being in sufficient amount to ensure neutralization of the valproic acid moiety of the divalproex sodium. The neutralized divalproex sodium solution is sprayed onto a pharmaceutically acceptable carrier, and processed to obtain divalproex sodium tablets.
Owner:ANDRX LABS
Solid Pharmaceutical Dosage Form
InactiveUS20110028456A1High drug loadingEasy to manufacturePowder deliveryBiocideValsartanTrenbolone
A pharmaceutical composition comprising a solid unit dosage form comprising: one or more of pharmaceutically active ingredients selected from valacyclovir, olanzapine, voriconazole, topotecan, artesunate, amodiaquine, guggulosterone, ramipril, telmisartan, tibolone, atorvastatin, simvastatin, amlodipine, ezetimibe, fenofibrate, tacrolimus, valgancyclovir, valsartan, clopidrogel, estradiol, trenbolone, efavirenz, metformin, pseudoephedrine, verapamil, felodipine, valproic acid / sodium valproate, mesalamine, hydrochlorothiazide, levosulpiride, nelfinavir, cefixime and cefpodoxime proxetil in combination with a water insoluble polymer and / or a water soluble polymer. Methods for making the pharmaceutical composition are also disclosed.
Owner:CIPLA LTD
Controlled release sodium valproate formulation
InactiveUS20050276850A1Increase release rateBiocideGranular deliveryValproic AcidControlled-Release Formulations
Disclosed is a controlled release formulation comprising valproic acid, pharmaceutically acceptable salt thereof, amide thereof, or derivative thereof.
Owner:ANDRX
Application of composite high-fat forage to construct non-alcoholic fatty liver disease rat model
The invention belongs to the field of animal experiment models, and discloses application of a composite high-fat forage to construct a non-alcoholic fatty liver disease rat model. The high-fat forage is composed of the following raw materials in percent by mass: 77.5% of a rat basic forage, 10% of egg, 10% of coconut oil, 2% of cholesterol, 0.5% of bile salt, and 500mg / kg / d of sodium valproate calculated according to the rat weight. After 8 weeks, rats all have typical non-alcoholic fatty live symptoms, and liver has a lot of fat accumulation along with inflammatory cell infiltration. The model establishing time is short, the success rate is high, and the composite high-fat forage is applicable to pathogenesis research induced by high-fat-diet combined medicines with liver-toxicity side effect, screening of related control measures and efficacy evaluation of treatment medicines.
Owner:NANJING GENERAL HOSPITAL NANJING MILLITARY COMMAND P L A +1
Method for rapidly sensitively detecting sodium valproate in blood
InactiveCN104111284AHigh speedSimple methodMaterial analysis by electric/magnetic meansSingle sampleIon-mobility spectrometry
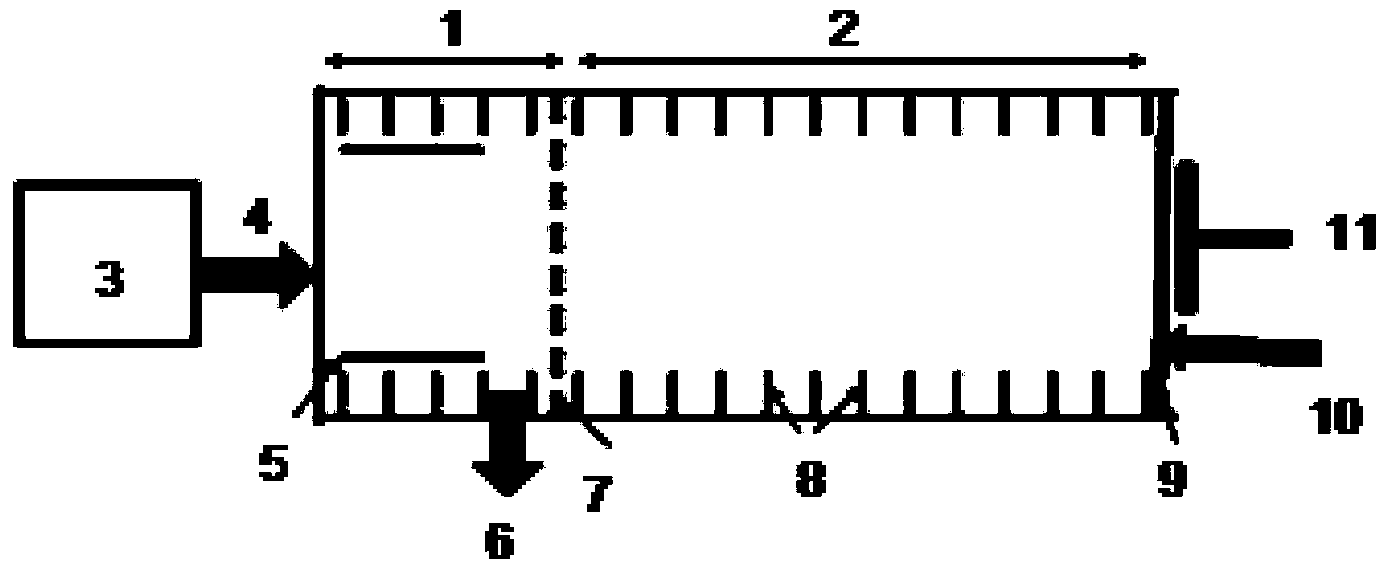
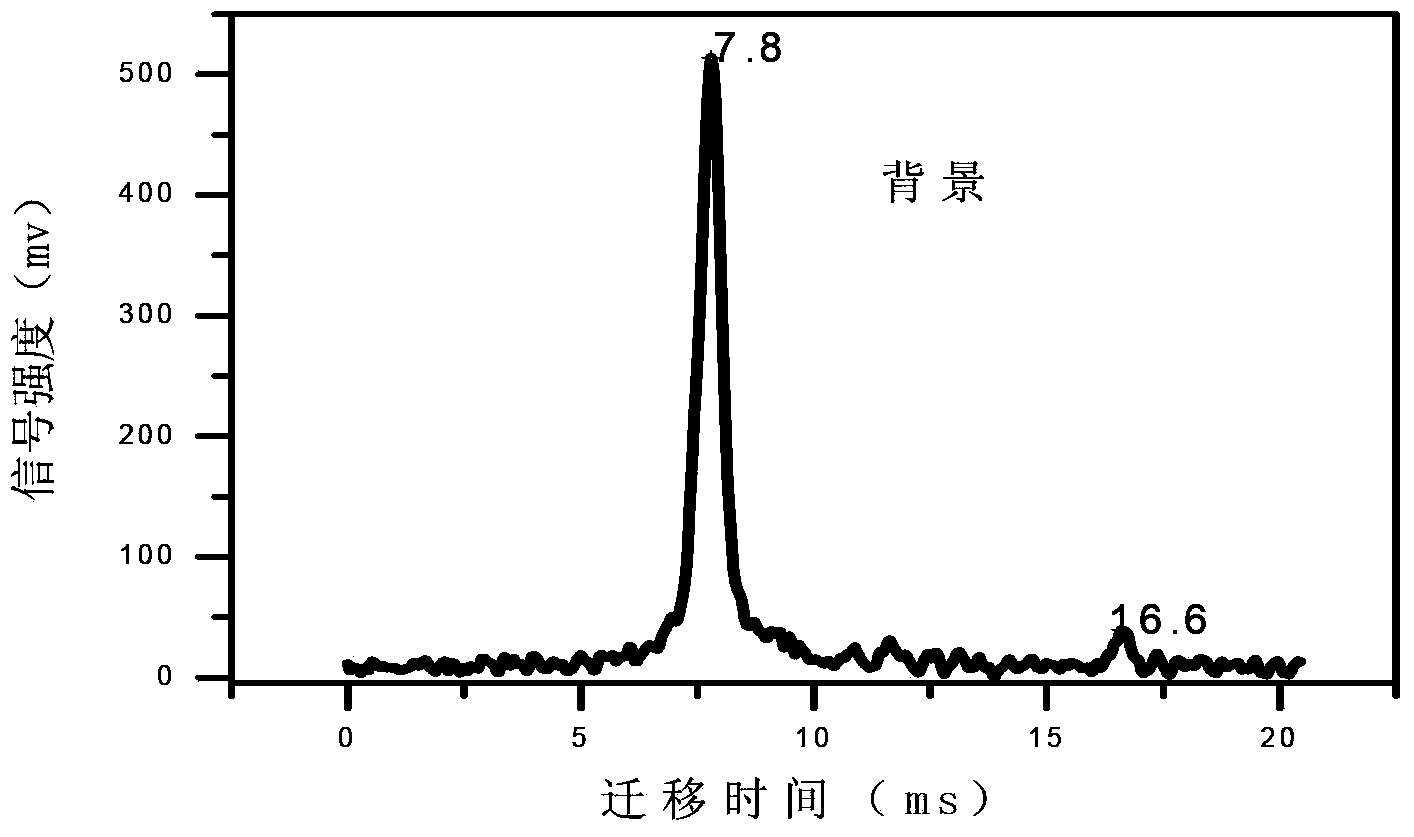
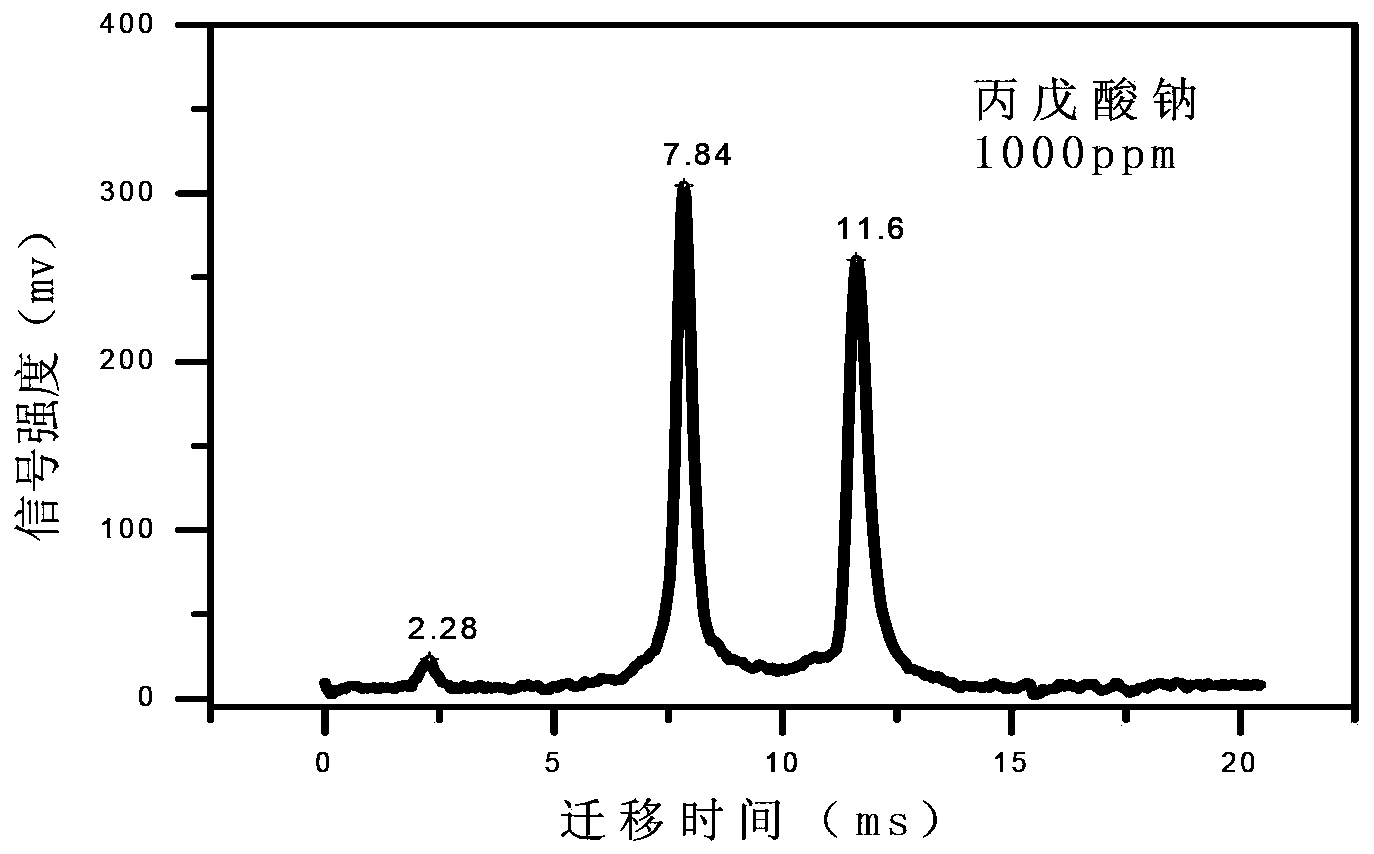
The invention discloses a method for rapidly sensitively detecting sodium valproate in blood. An ion migration spectrum technology is taken as a basic detection technology, an ion migration spectrometer is utilized as a detection means, a positive-ion high-voltage mode is employed, a halogen-lamp thermal desorption sampling manner is employed, and the method does not comprise any solvent extraction pretreatment. An apparatus employed in the method is stable and reliable, and the method is simple, rapid and efficient. The detection analysis time of a single sample is less than 20 S. The detection sensitivity is high, the measure detection limit can reach 1 mg / L, and the quantitative analysis concentration scope is 5-500 mg / L. The established qualitative quantitative analysis method satisfies the human-body medicine-administration concentration analysis scope (50-100 mg / L) of a doctor to an epilepsy patient. The detection method is widely applicable to clinical medicine-administration deep analysis, and helps to guide a doctor to clinically precise administrate a medicine.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Technique for preparing disodium valproate
InactiveCN101003476AShort cycleLow costCarboxylic acid salt preparationValproic AcidSodium divalproex
This invention discloses a process for preparing sodium divalproate. The process comprises: dissolving valproic acid and sodium valproate at a mol ratio of 1:1 in an appropriate solvent, and vacuum-distilling the solvent at 55-90 deg.C to obtain sodium divalproate. The process has such advantages as easy control, no byproducts, high product yield, high product quality, and low cost. The process can be used for mass production of sodium divalproate.
Owner:QINGDAO UNIV OF SCI & TECH
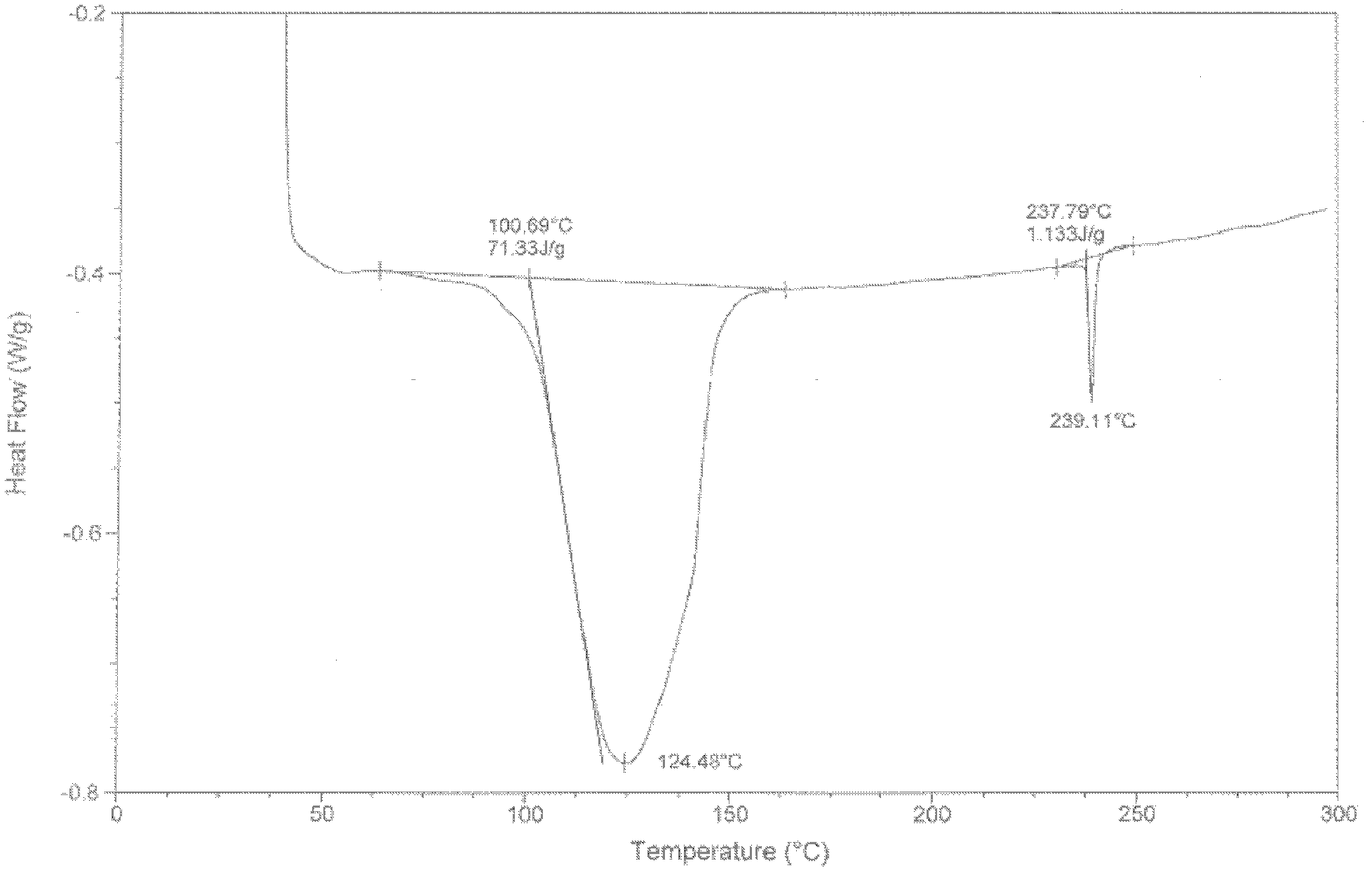
New crystal form for sodium valproate and preparation method and usage thereof
ActiveCN102079699AImprove securityGuaranteed curative effectNervous disorderAnhydride/acid/halide active ingredientsSolubilityX-ray
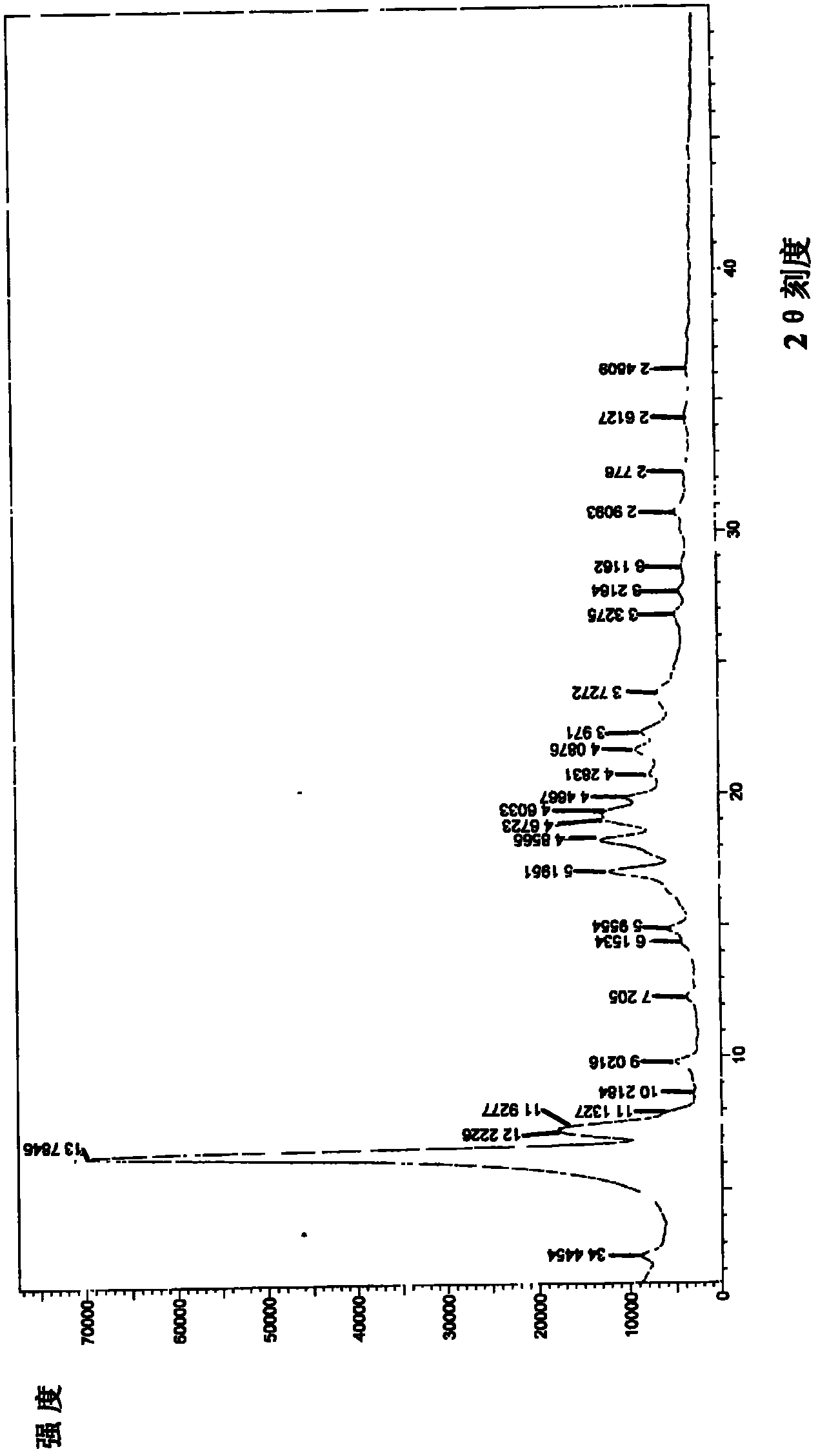
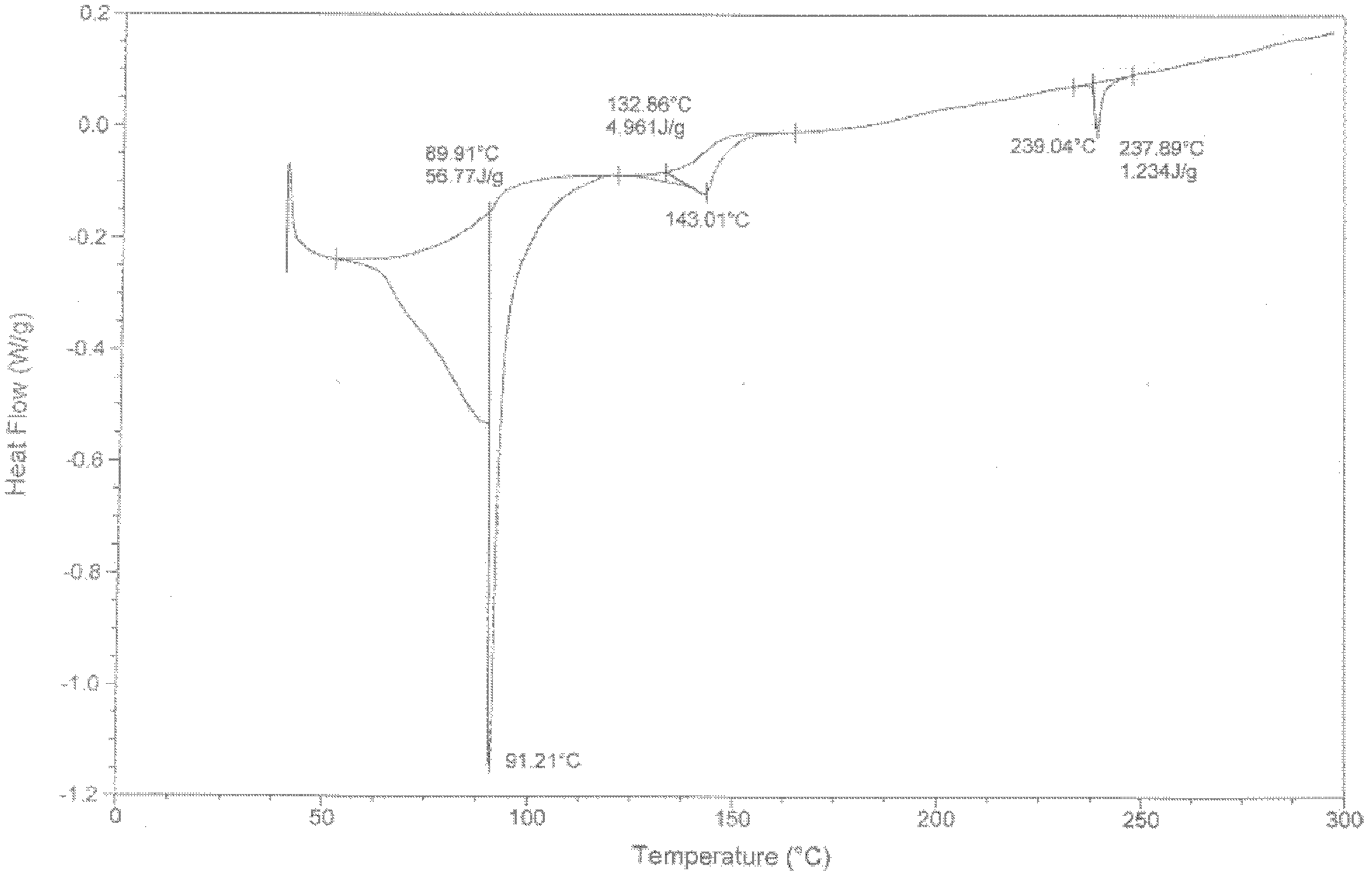
The invention provides a sodium valproate crystal form III. In a diffraction pattern of powder X-ray diffraction of a crystal, characteristic absorption peaks exist at diffraction angles (2 theta) of 5.40 DEG, 6.31+ / -0.1 DEG, 7.28 DEG, 76.86 DEG and 18.10 DEG. The invention also provides a preparation method of the crystal form and a medicinal composition containing the crystal form. The sodium valproate crystal from III has high solubility and controllability, is safe and stable and provides a new preparation choice for clinical application.
Owner:SICHUAN CREDIT PHARMA
Method for detecting sodium valproate content of blood through high performance liquid chromatography
InactiveCN103454369ACalculation contentEasy to operateComponent separationInternal standardPeak value
The invention relates to the technical field of biochemical and provides a method for detecting sodium valproate content of blood through high performance liquid chromatography. The method comprises the steps of preparing 37.6mug / ml of a sodium valproate standard solution, sodium valproate working solutions with various concentrations, 50.2mug / ml of a hexahyl carbonic acid internal standard solution, a blank sample, a normal human blood sample and a patient blood sample; putting into a chromatographic instrument to draw chromatograms, and judging whether the blood of a patient contains sodium valproate according to the chromatograms; then drawing a sodium valproate standard curve with the chromatographic instrument according to the chromatogram of the normal human blood sample, and calculating the sodium valproate content of the blood of the patient according to a peak sodium valproate value of the chromatogram of the patient blood sample, a peak value of the hexahyl carbonic acid internal standard solution and the sodium valproate standard curve. According to the method, the problems that the chromatograms contain multiple stray peaks, the sensitivity is low, the precision is low, the cost is high, and the sodium valproate standard curve cannot be drawn are effectively solved.
Owner:上海兰卫医学检验所股份有限公司
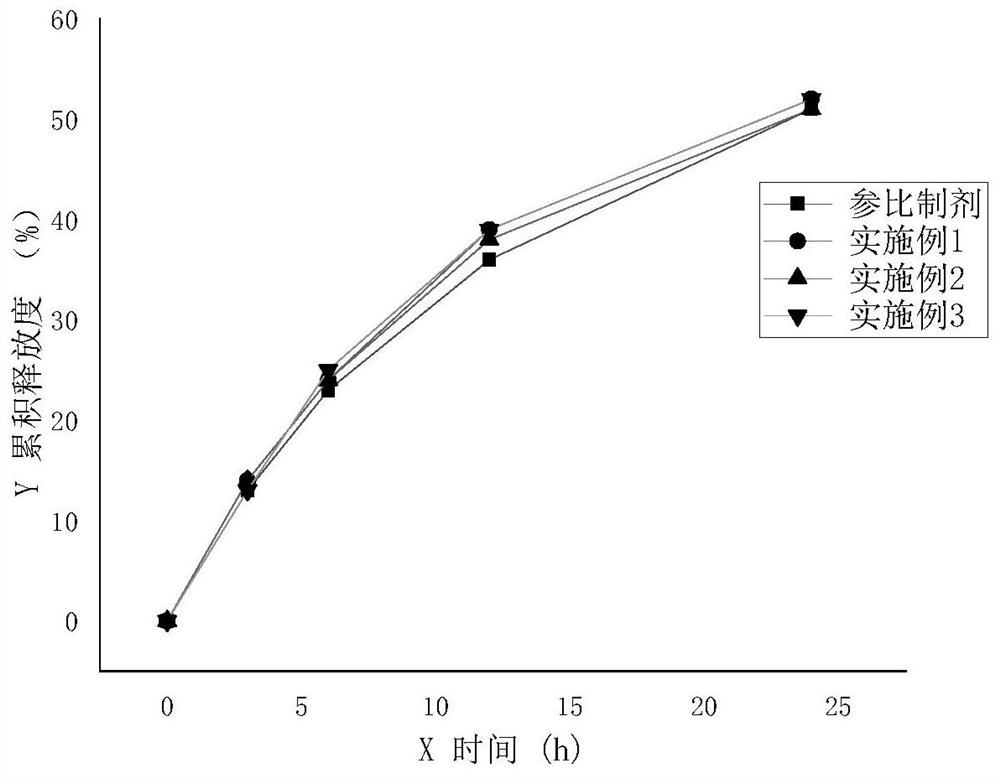
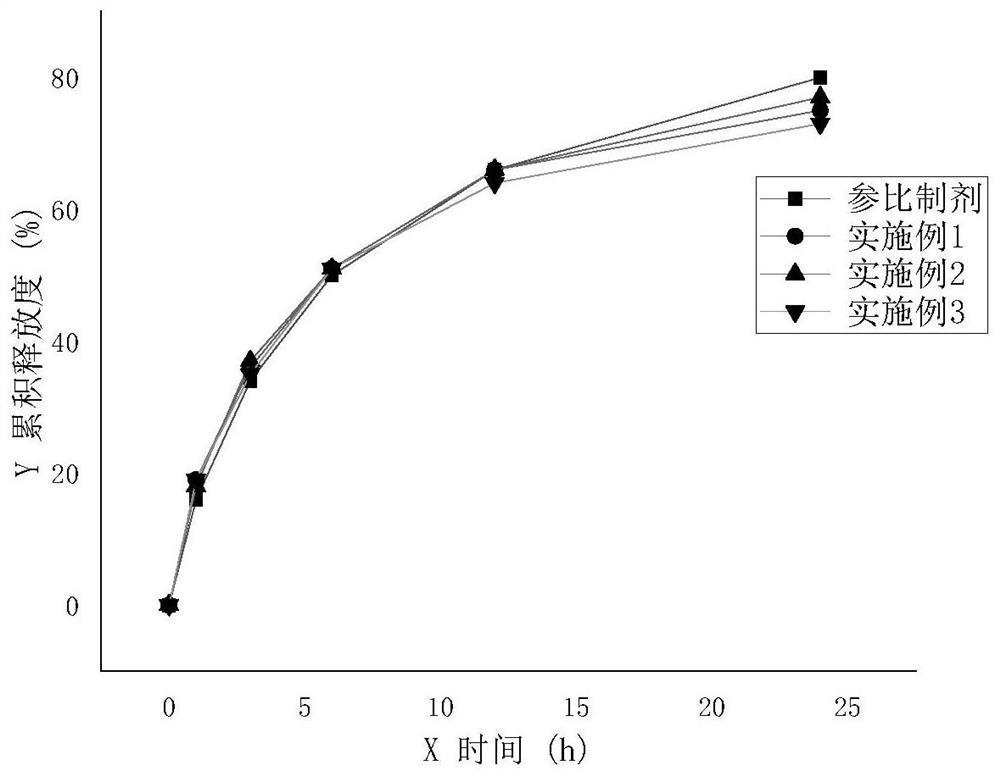
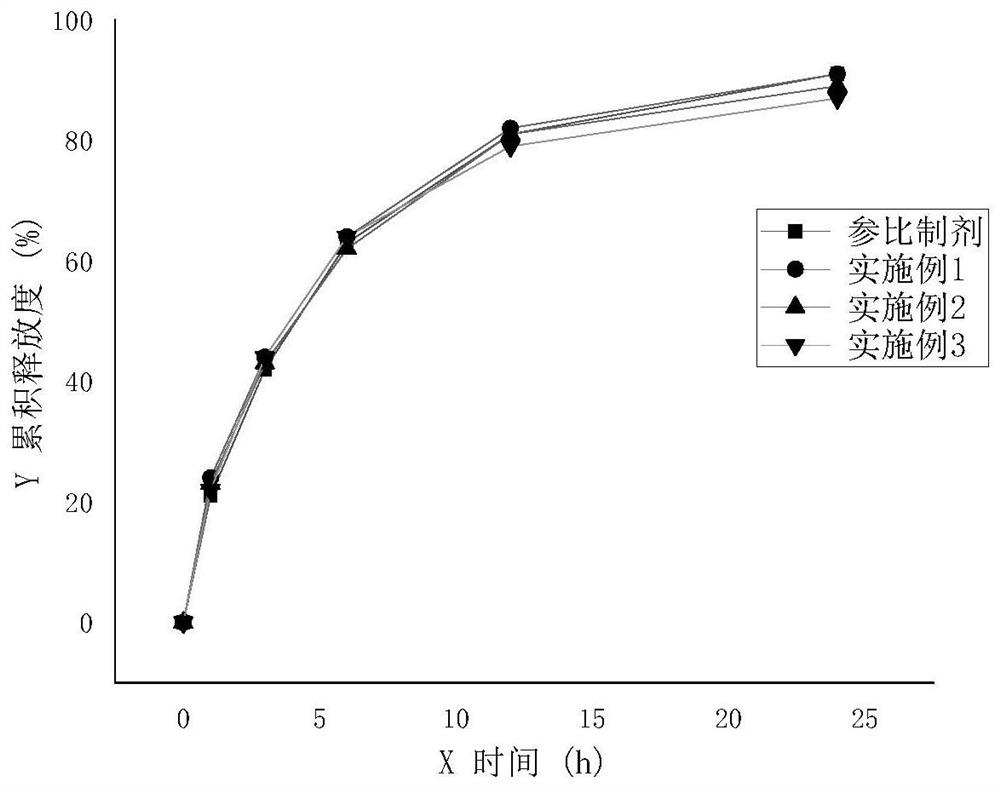
Sodium valproate sustained release tablet as well as preparation process and application thereof
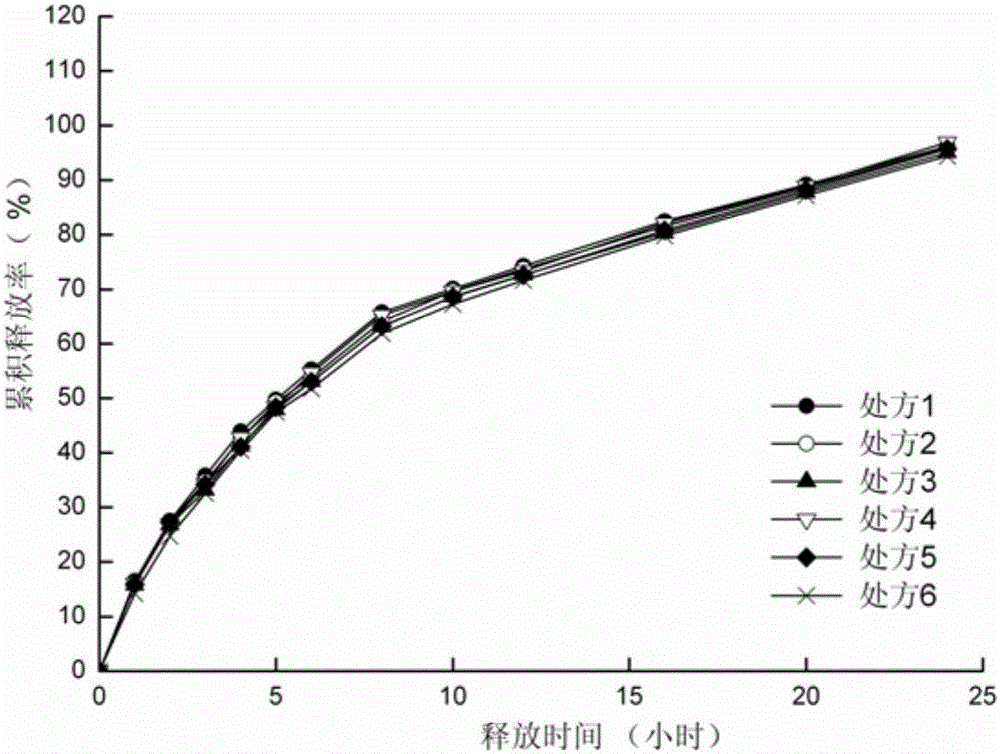
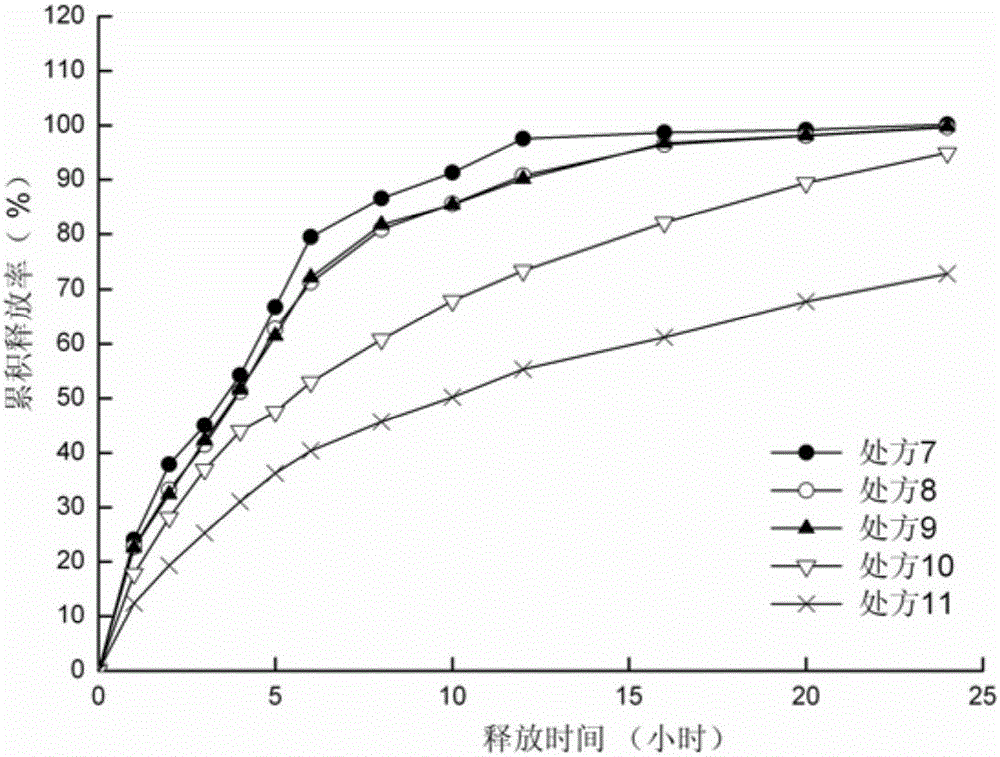
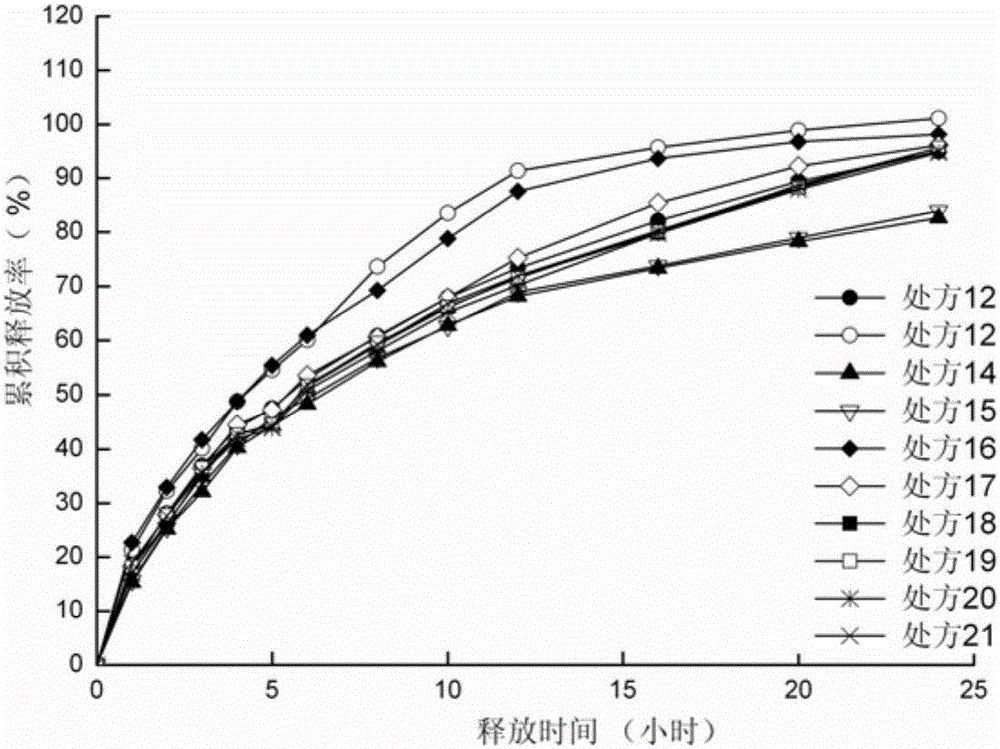
ActiveCN105012264ASimple preparation processLow equipment requirementsNervous disorderPharmaceutical delivery mechanismSustained Release TabletSide effect
The invention discloses a sodium valproate sustained release tablet and also discloses a prescription and preparation process of the sustained release tablet. The sustained release tablet can continuously and stably release drugs in 24 hours, so that more stable blood concentration is obtained after patients take the drug, side effects are reduced and the compliance of the patients is increased. The preparation process of the sustained release tablet is simple and is convenient for industrial production.
Owner:SICHUAN CREDIT PHARMA
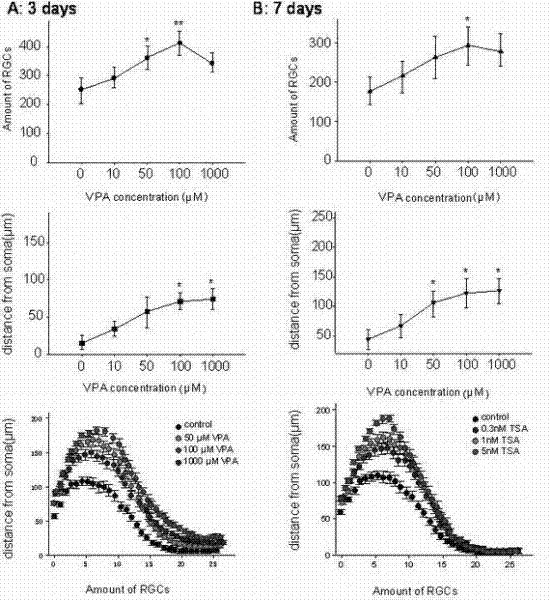
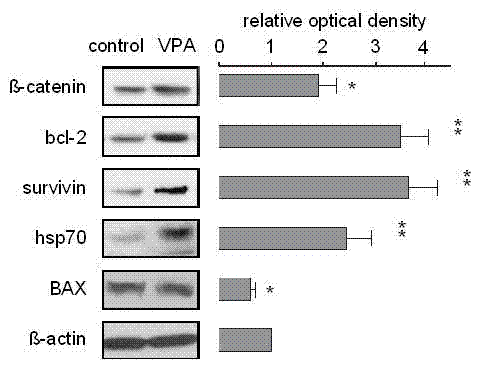
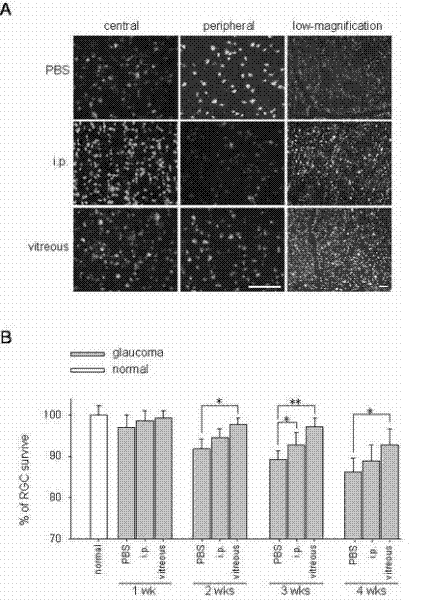
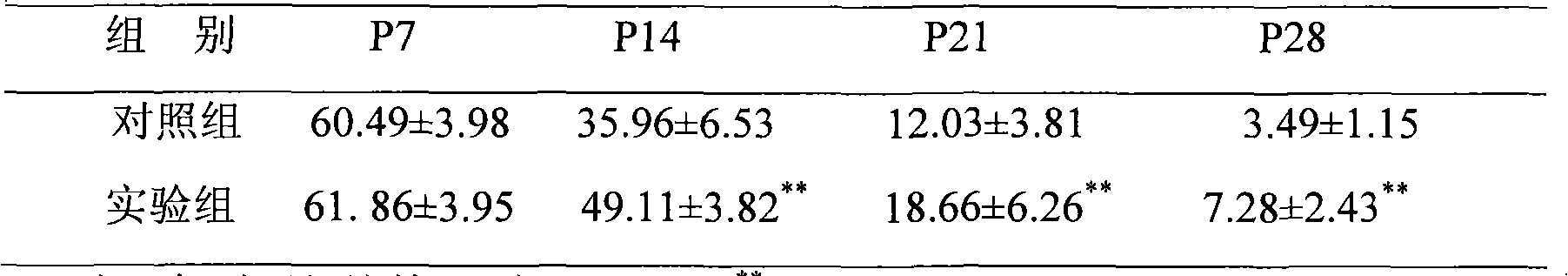
Application of sodium valproate in preparation of medicament for treating or improving optic nerve pathological changes of glaucoma
InactiveCN102218051AThrough highEasy to degradeSenses disorderAntinoxious agentsRetinal ganglionHigh intraocular pressure
The invention belongs to the field of pharmacy, and relates to application of sodium valproate in preparation of medicaments for treating or improving optic nerve pathological changes of glaucoma. The in-vitro cell culture experiment and in-vivo animal experiment indicate that sodium valproate with certain concentration can promote retina ganglionic cells (RGCs) cultured in vitro to survive and enhance the extension capacity of the axon, and can lower the expression of proapoptotic gene BAX and promote the RGCs to survive under the conditions of chronic high intraocular pressure, thereby relieving the damage of RGCs. The experiments prove that the sodium valproate can protect the RGCs in the aspects of multiple pathogeneses of glaucoma optic nerve pathological changes, and further more, can be used as an active ingredient for preaprating medicaments for treating glaucoma optic nerve pathological changes, especially medicaments for protecting RGCs. The invention has important application value and favorable social and economic benefits in clinical treatment of glaucoma.
Owner:EYE & ENT HOSPITAL SHANGHAI MEDICAL SCHOOL FUDAN UNIV
Medicine for treating dumps emotional handicap type disease
InactiveCN101081272AEasy to takeImprove compliancePowder deliveryNervous disorderDiseaseTypes diseases
The medicine for treating depression and other mental disturb diseases is prepared with chlorimipramine, sulpiride, sodium valproate, borneol and Chinese herbal medicine extractum prepared with nutagrass flatsedge rhizome, arisaema with bile, wild jujube seed and other Chinese medicinal materials. The medicine consists of chemically synthetic medicine and Chinese herbal medicine component, and has synergistic treating effect, less toxic side effect, and good patient's compliance.
Owner:张宝山
Application of sodium valproate during preparing medicament for treating retina disease
InactiveCN101584686ADelayed degenerationAnhydride/acid/halide active ingredientsCardiovascular disorderDiseaseActive component
The invention discloses an application of sodium valproate during preparing medicament for treating retina disease, the treating of the retina disease is to protect the retina photoreceptor cell. It is proved that, the sodium valproate is applied to an animal model with pigmentary degeneration of retina which is one kind of the retina disease, the action for acting against the apoptosis of the photoreceptor cell, the progressive degrading of the photoreceptor cell can be delayed, the sodium valproate can be used as active component to prepare the retina disease neural protection medicament, especially to prepare the medicament for protecting the photoreceptor cell, and the good effect can be reached.
Owner:PEKING UNIV FIRST HOSPITAL
Divalproex sodium tablets
A process for preparing divalproex sodium tablets is provided. The process comprises preparing a neutralized divalproex sodium solution by combining divalproex sodium, having a sodium valproate and a valproic acid moiety, with an aqueous solvent and a base, e.g., sodium hydroxide, the base being in sufficient amount to ensure neutralization of the valproic acid moiety of the divalproex sodium. The neutralized divalproex sodium solution is sprayed onto a pharmaceutically acceptable carrier, and processed to obtain divalproex sodium tablets.
Owner:ANDRX
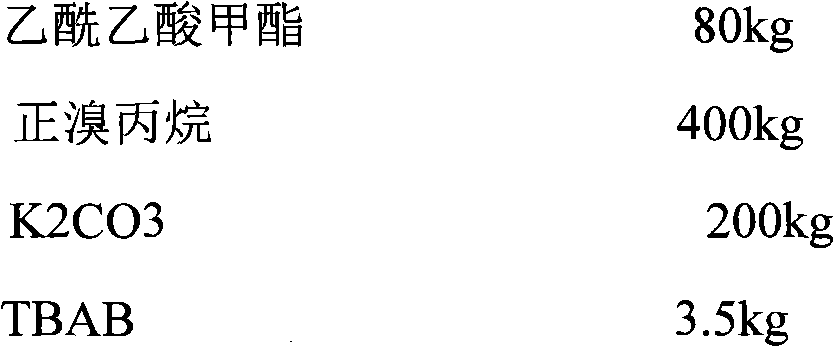
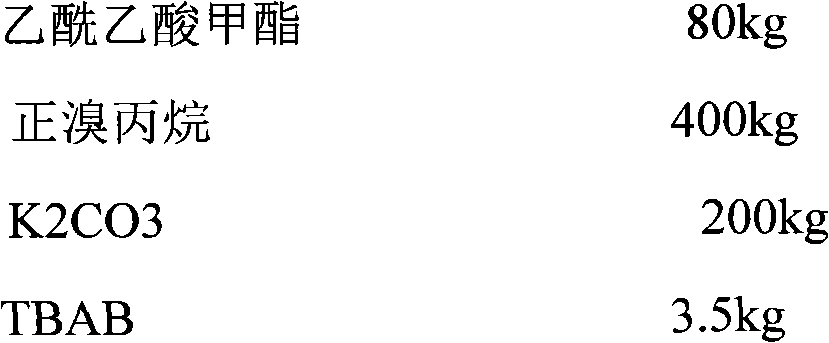
Synthesis process for sodium valproate
InactiveCN105622390AIncrease conversion rate per passHigh yieldCarboxylic acid salt preparationGas phaseValproic Acid
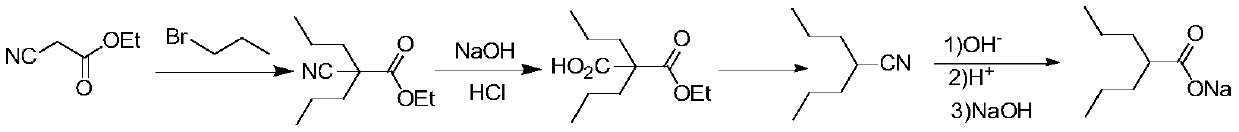
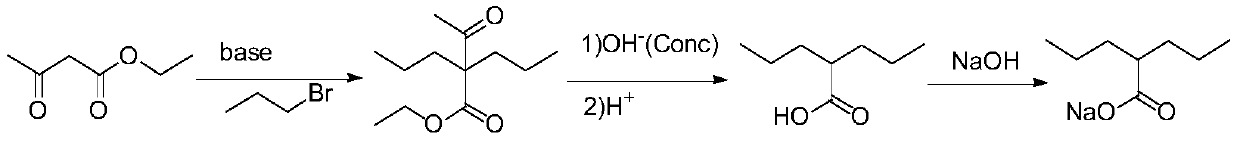
The invention discloses a synthesis process for sodium valproate. The synthesis process comprises the following steps: mutually dissolving diethyl malonate and 1-bromopropane, slowly adding the obtained mixture into an ethanol solution of sodium ethoxide at a certain temperature, carrying out heating and reflux for 2 h, recovering ethanol until temperature is 110 DEG C, carrying out cooling to less than 80 DEG C, adding a certain amount of water to dissolve sodium bromide, carrying out layering to obtain a plurality of layers, then adding an aqueous sodium hydroxide solution with a concentration of 15 to 30%, carrying out hydrolysis at 60 to 70 DEG C for 3 h, then carrying out heating to recover ethanol until a gas phase temperature is 99 DEG C, carrying out cooling to less than 80 DEG C, adding hydrochloric acid for neutralization and acidifying, adding crude valproic acid to dissolve dipropylmalonic acid so as to obtain mixed acid and subjecting the mixed acid to slow heating and decarboxylation at 110 to 160 DEG C for production of crude valproic acid; and subjecting the crude valproic acid to rectification and refining, adding a certain amount of the aqueous sodium hydroxide solution for neutralization, adding toluene for reflux to bring out water, thereby allowing sodium valproate to dehydrate and crystallize and then successively carrying out filtering, washing with chloroform and drying so as to obtain finished sodium valproate. The process is safe and environment-friendly, produces good-quality sodium valproate, has low cost and is suitable for industrial production.
Owner:QINGDAO SHOUTAI AGRI SCI & TECH CO LTD
Brightening agent for composite copper plating
ActiveCN108265317AImprove densification performanceGood dispersionCopper platingGlycidyl methacrylate
The invention relates to a brightening agent for composite copper plating and belongs to the technical field of electroplating additives. According to the brightening agent, firstly, maleic anhydrideand acrylic acid are used as raw materials; under the action of ammonium persulfate, polymerization is carried out to form a copolymer; in a polymerization process, sodium valproate and vinylphosphonic acid are added, and are grafted into the copolymer under the catalysis effect of strong acidic styrene cation exchange resin; then N,N-dimethylformamide, anisole, glycidyl methacrylate and the likeare used as raw materials to carry out polymerization; two polymers have good dispersion performance, and copper ions can be uniformly dispersed on the surface of a matrix to carry out copper plating;added benzalacetone and salicylaldehyde can slow down the growth speed of a crystal, a crystal nucleus formation speed is improved and the compactness of crystal grains of a copper plated layer is improved; a surfactant is added and the generation of needle holes is reduced under the action of an auxiliary agent; meanwhile, the binding performance of the plated layer and the matrix is reinforcedby utilizing the effect of the auxiliary agent.
Owner:连云港市赣榆金成镍业有限公司
Bi-path detecting card capable of simultaneously detecting carbamazepine and sodium valproate and detecting method of same
InactiveCN102331499AEasy to manufactureLow detection costMaterial analysisAntiepileptic AgentsEngineering
The invention provides a bi-path detecting card capable of simultaneously detecting carbamazepine and sodium valproate and a detecting method of the same, and belongs to the technical field of the monitoring on the blood concentration of antiepileptic drug. In the invention, a detecting window and a sample adding hole are arranged on the surface of a shell of the detecting card; a testing bar is arranged in the shell; a nitrocellulose film is adhered to the middle part of a backing board of the testing bar; a water absorbent film is adhered to one end of the backing board; a sample pad is adhered to the other end of the backing board; two segments of colloidal gold films are arranged between the water absorbent film and the sample pad in an adhesive manner; the colloidal gold films are respectively glass fiber films containing colloidal gold markers of monoclonal antibodies resistant to the carbamazepine and the sodium valproate; three separated imprint display strips are transversely arranged on the nitrocellulose film, and include a detecting strip containing carbamazepine protein conjugate, an another detecting strip containing sodium valproate protein conjugate and a quality control strip containing antibodies resistant to a rabbit or a rat; the sample pad is just opposite to the sample adding hole; and the nitrocellulose film is just opposite to the detecting window. The detecting card can be used for simultaneously detecting components of two anticonvulsants in a serum sample, and has the advantages of saved cost for detection, convenience for use, quickness in detection, high sensitivity and accuracy in result.
Owner:无锡安迪生物工程有限公司
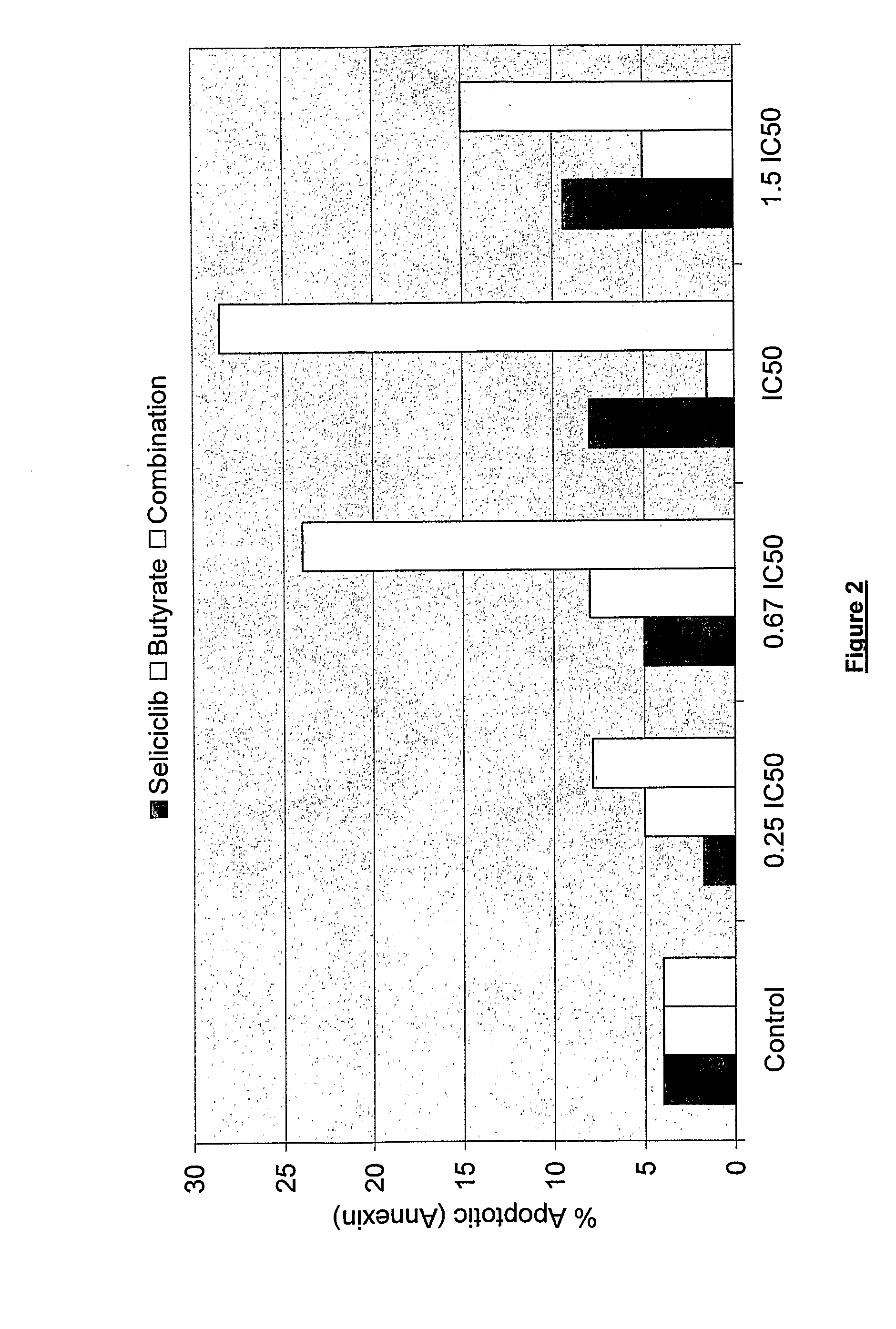
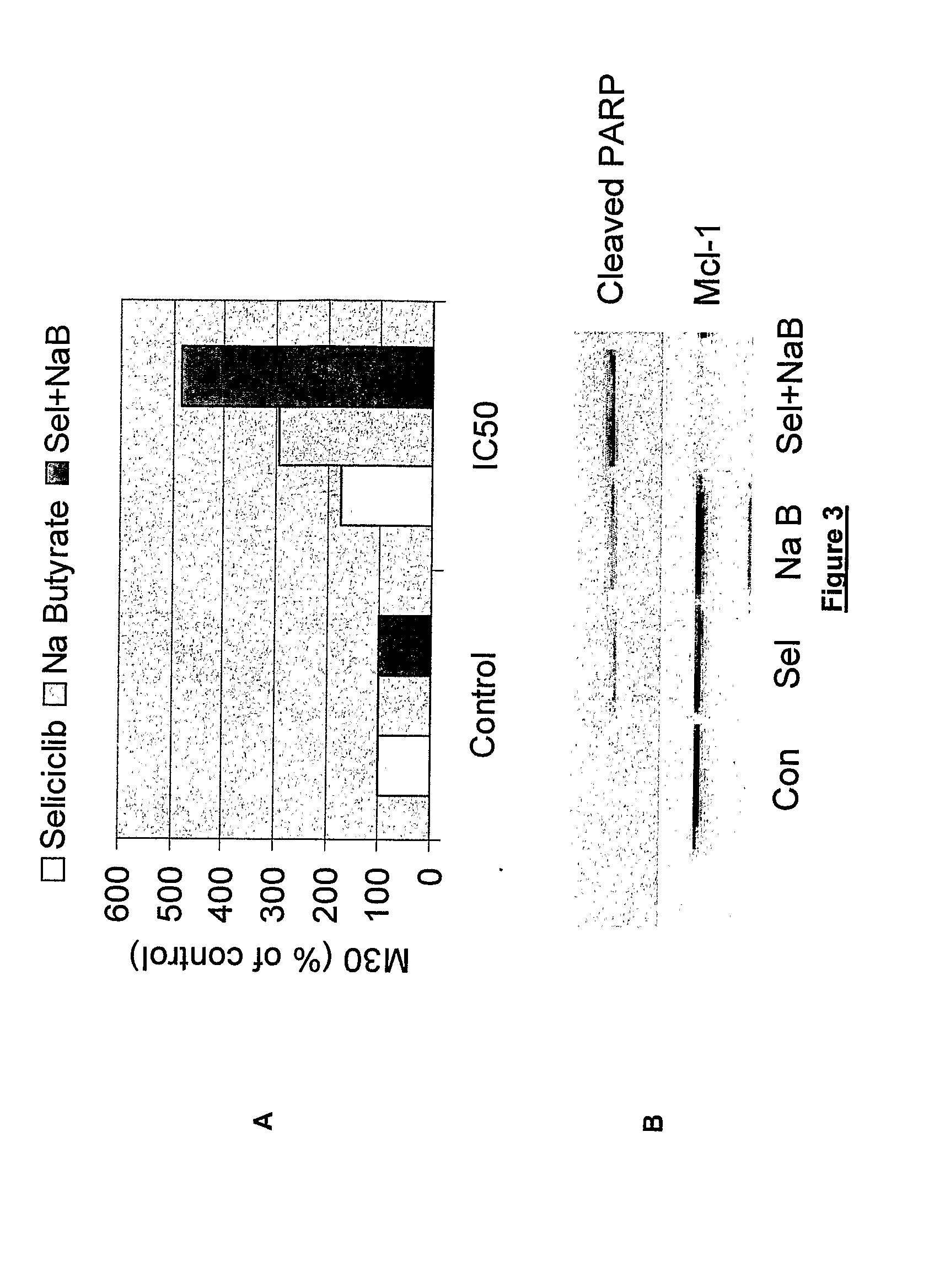
Combination of roscovitine and a hdca inhibitor to treat proliferative diseases
A first aspect of the invention relates to a combination comprising roscovitine, or a pharmaceutically acceptable salt thereof, and a HDAC inhibitor selected from sodium butyrate, or a prodrug thereof, suberoylanilide hydroxamic acid (SAHA), sodium valproate and trichostatin A (TSA). A second aspect of the invention relates to a pharmaceutical product comprising roscovitine, or a pharmaceutically acceptable salt thereof, and a HDAC inhibitor selected from sodium butyrate, or a prodrug thereof, suberoylanilide hydroxamic acid (SAHA), sodium valproate and trichostatin A (TSA) as a combined preparation for simultaneous, sequential or separate use in therapy. A third aspect of the invention relates to a method for treating a proliferative disorder, said method comprising simultaneously, sequentially or separately administering roscovitine, or a pharmaceutically acceptable salt thereof, and a HDAC inhibitor selected from sodium butyrate, or a prodrug thereof, suberoylanilide hydroxamic acid (SAHA), sodium valproate and trichostatin A (TSA) to a subject.
Owner:CYCLACEL
Sodium valproate crystal form as well as preparation method and application thereof
ActiveCN102603510AQuality improvementSignificant effectNervous disorderAnhydride/acid/halide active ingredientsX-rayCurative effect
The invention discloses a sodium valproate crystal form II as well as a preparation method and application thereof, wherein characteristic absorption peaks appear at diffraction angles of (2theta)=6.3-6.5 DEG, 7.12-7.32 DEG, 7,3-7.5 DEG, 16.95-17.15 DEG, 18.15-18.35 DEG, 18.88-19.08 DEG and 19.17-19.37 DEG in a powder x-ray diffraction pattern of the crystal. The sodium valproate crystal form II provided by the invention has the advantages that the hygroscopicity is significantly reduced and lower water content of the product is guaranteed to ensure that the quality of the sodium valproate is more stable in storage period; while the hygroscopicity is reduced, the dissolution time of the product is significantly shortened and the dissolvability of the product is improved to ensure that the curative effect and very good safety of the sodium valproate are guaranteed in clinical application, the injection pain is also significantly relieved, compliance of patients is increased during treatment and very good clinical effect of the treatment is obtained.
Owner:SICHUAN CREDIT CHEMWERTH PHARMACEUTICAL CO LTD
Composition comprising prostaglandin derivatives and ophthalmic liquid formulation comprising the composition
ActiveCN109172580AReduce concentrationReduce usageSenses disorderPharmaceutical delivery mechanismSodium divalproexMagnesium Valproate
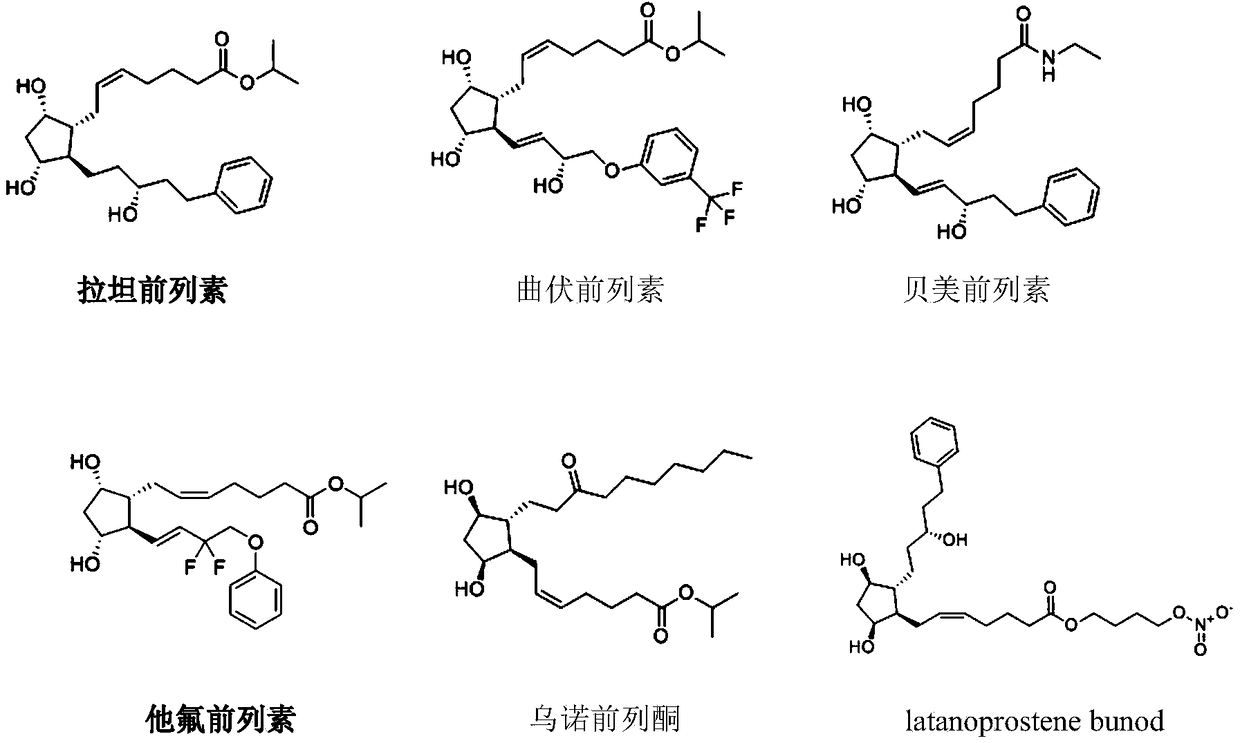
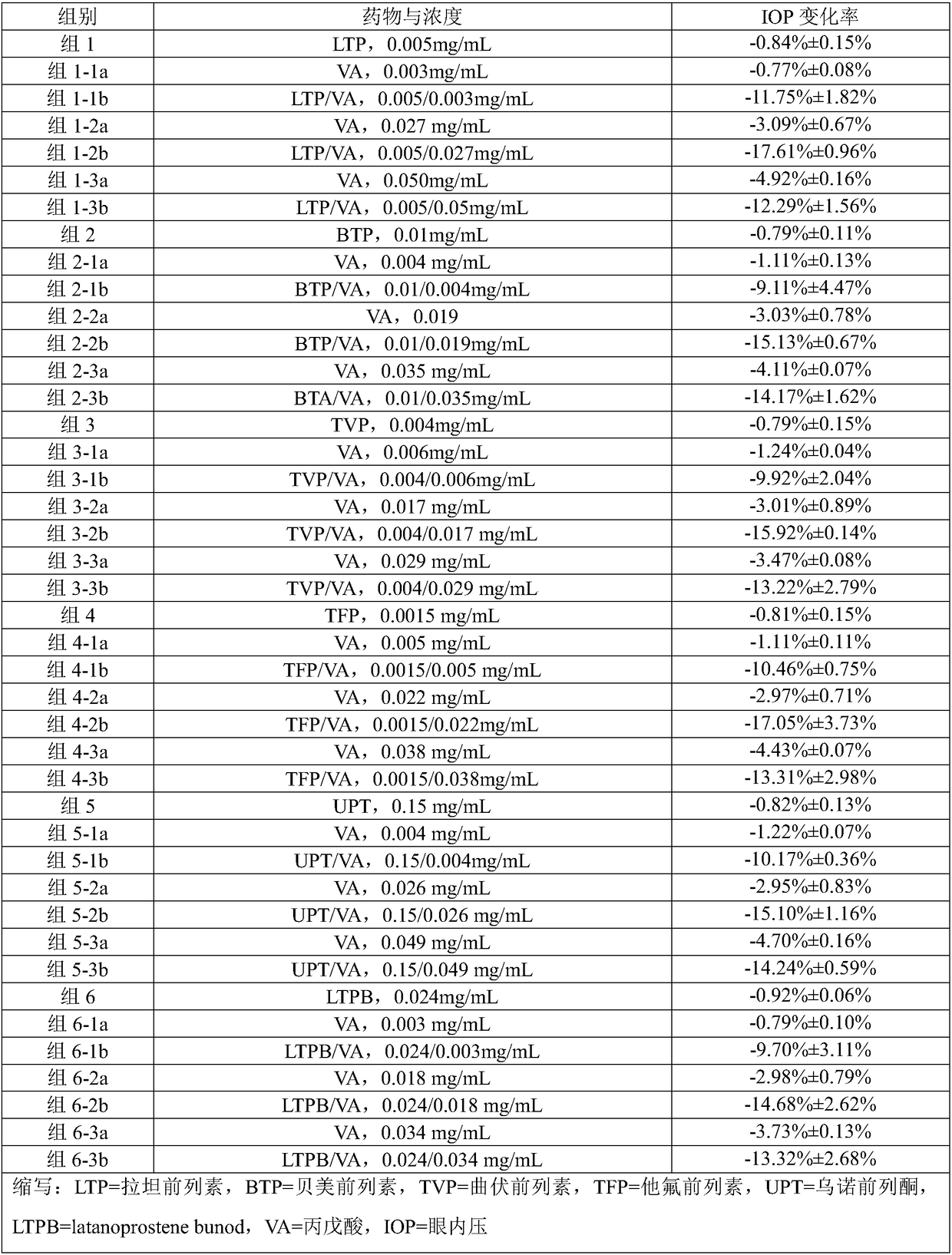
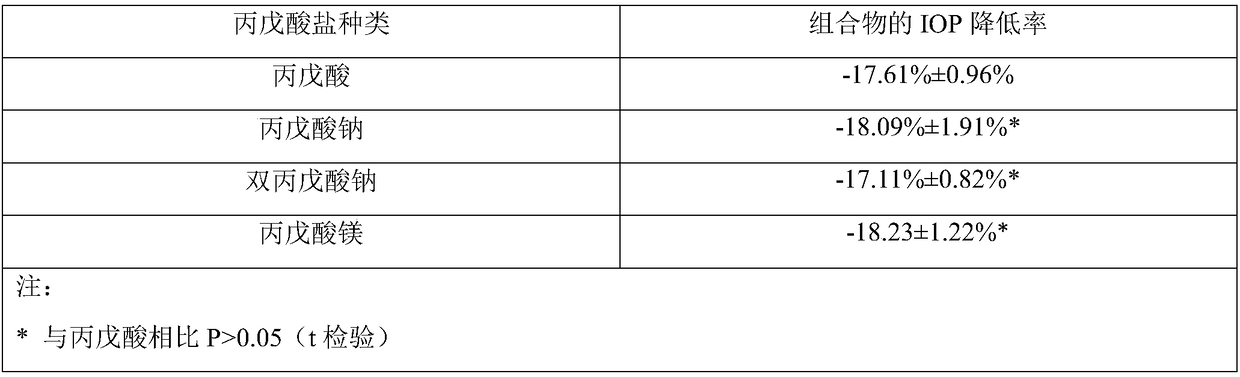
The invention provides a composition comprising prostaglandin derivatives and valproic acid or a pharmaceutically acceptable salt thereof and an ophthalmic liquid formulation comprising the composition, wherein the prostaglandin derivative is preferably one of latanoprost, bemeprost, travoprost, taflurane, unoprost and latanoprostene, and valproic acid or a pharmaceutically acceptable salt thereofis preferably one of valproic acid, sodium valproate, sodium divalproate and magnesium valproate. The composition provided by the invention can synergistically reduce intraocular pressure, avoid theuse of cosolvent, and improve the safety of medicament.
Owner:ZHONGSHAN WANHAN PHARM CO LTD
Sodium valproate injection, preparation method and applications thereof
InactiveCN105640873AEnsure safetyOvercome the uncontrollable defects of clinical drug safetyNervous disorderPharmaceutical delivery mechanismActive componentMedicine
The invention provides a sodium valproate injection, which is prepared from sodium valproate (active component), a pH adjuster, and injection water, wherein the pH of the injection is 7.9 to 8.1. The invention also provides a preparation method of the injection. The provided sodium valproate injection does not contain any stabilizer or antiseptic, so the defect that in the prior art, the safety of auxiliary materials is uncontrollable in clinic is overcome, and thus the safety of injection is guaranteed. Moreover, the injection is nonirritant and is insoluble in blood, the clinical safety can be ensured, the manufacturing cost of the sodium valproate injection is low, and the safety is excellent. Compared with sodium valproate injections, which contains or does not contain a stabilizing agent and is in other pH range, the experiment data of six month accelerated stability and twelve month long term stability of prepared sodium valproate injection is better.
Owner:SICHUAN CREDIT PHARMA
Preparation method of sodium valproate
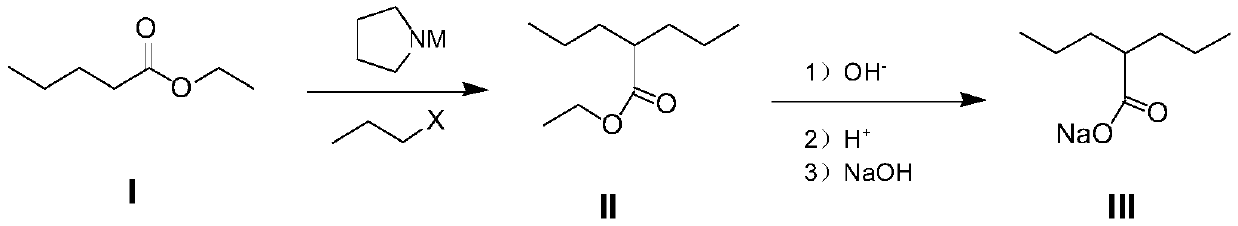
PendingCN111349003AShort reaction pathHigh yieldOxygen-containing compound preparationOrganic compound preparationHalopropanePyrrole
The invention provides a preparation method of sodium valproate, and belongs to the technical field of medicine synthesis. The method comprises the following steps: by taking ethyl valerate as a raw material, adding a methyl tert-butyl ether solution of a pyrrole metal reagent into an ether solution of ethyl valerate, then adding halopropane, carrying out an alkylation reaction, adding a weakly acidic solution in a dropwise manner to terminate the reaction after the reaction is finished, and washing with water to obtain an intermediate product; and adding a sodium hydroxide solution into the alcohol solvent of the intermediate product, carrying out a saponification reaction, and purifying to obtain sodium valproate after the saponification reaction is finished. The method is short in reaction route, high in total yield, easily available in raw materials, low in cost, high in operability and suitable for industrialization. The total molar yield of sodium valproate prepared by the methodis greater than or equal to 86.0%, and the purity of the final product is greater than or equal to 99.5%.
Owner:SICHUAN CREDIT PHARMA
Novel crystal form of sodium valproate, and preparation method and application thereof
ActiveCN102531878AQuality improvementReduce quality problemsNervous disorderOrganic chemistryX-rayMoisture absorption
The invention discloses a crystal form I of sodium valproate. In an X-ray powder diffraction pattern of the crystal, characteristic absorption peaks appear when a diffraction angle (2 theta) is equal to 5.54+ / -0.1 degrees, 6.64+ / -0.1 degrees, 20.90+ / -0.1 degrees, 24.38+ / -0.1 degrees and 26.13+ / -0.1 degrees. The invention also discloses a preparation method and application of the crystal form I. According to the crystal form I of the sodium valproate, the moisture absorption is obviously reduced, low water content of the product is ensured, and the sodium valproate has stable quality in a storage period; when the moisture absorption is reduced, the dissolution time of the product is obviously shortened, the resolubility of the product is improved, the treatment effect and high safety of the sodium valproate in clinical treatment are ensured due to high resolubility, the pain during injection is relieved, the compliance of the patient in a treatment period is improved, and a good clinical effect is achieved.
Owner:SICHUAN CREDIT CHEMWERTH PHARMACEUTICAL CO LTD
Preparation method of sodium valproate sustained release tablet
ActiveCN113304117ASolve the sticking problemAvoid problems with lump formationNervous disorderPharmaceutical non-active ingredientsProlonged-release tabletValproic Acid
The invention discloses a preparation method of a sodium valproate sustained release tablet, and belongs to the technical field of medicines. The sodium valproate sustained-release tablet disclosed by the invention consists of active medicines, namely valproic acid, sodium valproate, a flavoring agent, a framework material, a retardant, a lubricant, an adsorbent and a gastric-soluble coating premix. According to the preparation method of the sodium valproate sustained release tablet, the problem that sodium valproate in the product is not beneficial to industrial production due to extremely high hygroscopicity is solved, the hygroscopicity characteristic of the sodium valproate is utilized, the humidity in a fluidized granulator is increased to form granules in the fluidization process, the prepared granules have good flowability and tabletability, and the prepared product has good stability, has good similarity between in-vitro dissolution and original dissolution curves, and is suitable for large-scale production.
Owner:YANGTAI PHARMA SHANDONG
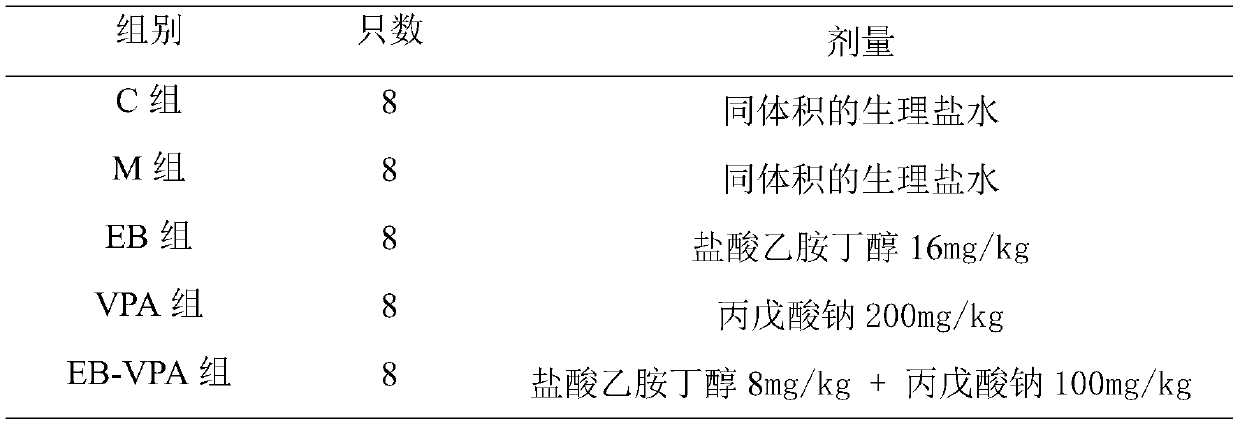
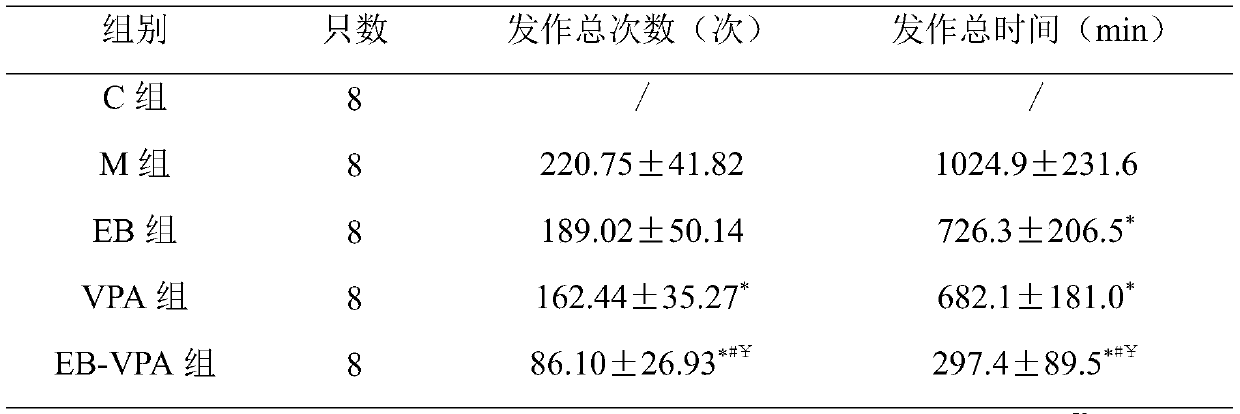
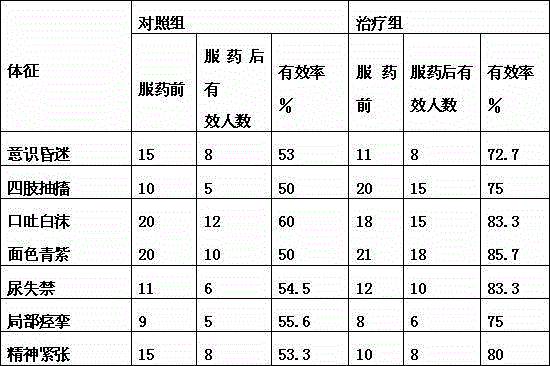
Pharmaceutical composition for treating intractable epilepsy and application thereof
ActiveCN109966277AHigh dependenceSmall toxicityNervous disorderAnhydride/acid/halide active ingredientsSide effectActive component
The invention discloses a pharmaceutical composition for treating intractable epilepsy and application thereof. The active components of the pharmaceutical composition comprise sodium valproate or divalproex sodium and ethambutol or medicinal salt thereof. The pharmaceutical composition has the advantages that the fact that low-dose ethambutol hydrochloride has an anti-epileptic effect is discovered for the first time, the ethambutol hydrochloride is combined with the sodium valproate or divalproex sodium to treat the intractable epilepsy, the pharmaceutical composition is evident in anti-epileptic effect, and the toxic and side effects of the pharmaceutical composition are lowered evidently.
Owner:AFFILIATED HOSPITAL OF JINING MEDICAL UNIV
Divalproex sodium sustained release pellets and preparation method thereof
InactiveCN104352445AReduce absorption rateStable absorptionNervous disorderGranular deliverySustained release pelletsAdhesive
The invention provides divalproex sodium sustained release pellets. The divalproex sodium sustained release pellets comprise medicine-containing pellets and coating layers, wherein the medicine-containing pellets are coated by the coating layers; the medicine-containing pellets comprise 250mg of divalproex sodium, 70mg of hollow pellet cores, 60-110mg of filling agent, 18-68mg of lubricating agent and 2-10mg of adhesive; the coating layers comprise 45-225mg of Eudragit NE30D and 7-68mg of talcum powder. A preparation method of the divalproex sodium sustained release pellets comprises the following processes: 1. material preparation; 2. mixing; 3. preparation of the adhesive; 4. preparation of the pellets; 5. preparation of a coating agent; 6. coating; 7. filling; 8. aluminium-plastic packaging and preparation of finished products. The divalproex sodium sustained release pellets used for treating epilepsy and mania and the preparation method have the beneficial effects that as the two kinds of advanced technologies, namely novel sustained release preparations and pellet preparations, are adopted, the divalproex sodium sustained release pellets have stable treatment effects and higher bioavailability and have the advantages of good medicine stability, convenience in packaging, transportation and storage, and the like; the preparation method is simple and practicable and is suitable for industrial production.
Owner:HARBIN SHENGJI PHARMA
Pour point depressant for crude oil, preparation method for pour point depressant and application of pour point depressant
InactiveCN105368432AChemically stableNo irritating smellPipeline systemsDrilling compositionEthylenediaminePhosphate
The invention discloses a pour point depressant for crude oil, a preparation method for the pour point depressant and application of the pour point depressant. The pour point depressant is prepared from the following ingredients in parts by weight: 2-15 parts of sodium 1,4-bis(1,3-dimethylbutyl) sulphonatosuccinate, 4-16 parts of polyvinyl pyrrolidone, 4-13 parts of hexadecyl dimethyl tertiary amine, 5-19 parts of sodium valproate, 5-18 parts of ammonium oxalate, 1-11 parts of nano-scale palladium borate, 400-600 parts of 10ppm-250ppm polyvinyl acid, 3-15 parts of 4-(1-methylethyl)cyclohexadiene-1-ethanol formate, 2-15 parts of polyacrylamide, 7-21 parts of cross-linker, 2-18 parts of vinylethylene carbonate, 2-12 parts of 4,4'-dimethoxytrityl chloride, 1-4 parts of 3,5-dibromomethyl toluene and 5-7 parts of sodium ethylenediamine tetramethylene phosphate. The pour point depressant for the crude oil, disclosed by the invention, is efficient and is stable in chemical property, and under the condition of low temperature, wax crystals cannot be precipitated when high-wax crude oil is transported by a pipeline; and the viscosity of the high-wax crude oil cannot be improved along with the drop of temperature, and the rheological property of the high-wax crude oil is not changed along with temperature drop.
Owner:XUZHOU UNIV OF TECH
Double sodium valproate orally disintegrating tablets and preparation method thereof
InactiveCN101310710AReasonable formulaGood compatibilityNervous disorderPill deliveryOrally disintegrating tabletCross-linked polyethylene
The invention discloses a divalproex sodium orally disintegrating tablet and a preparation method thereof. The principal medicine of the orally disintegrating tablet is divalproex sodium and the accessories are microcrystalline cellulose, mannitol, lactose, polyacrylic resin II, cross linked polyvinylpyrrolidone, sodium cyclamate, menthol and aerosil. The divalproex sodium orally disintegrating tablet of the invention can effectively cure falling sickness and vesania, can prevent hemicrania and has the advantages of convenient taking, good taste, fast disintegration, quick absorption and high biological availability. The divalproex sodium orally disintegrating tablet provides convenience for old people, children or patients having difficulty in swallowing and people having inconvenience to get water.
Owner:QINGDAO UNIV OF SCI & TECH
Sodium valproate and preparation process thereof
ActiveCN102579849AHigh yieldThe group is rational and orderlyNervous disorderAnhydride/acid/halide active ingredientsCelluloseSalvia miltiorrhiza
The invention relates to compound sodium valproate and a preparation process thereof. The sodium valproate is prepared by raw materials including sodium valproate 0.3 part, salvia miltiorrhiza 30 parts, pepper 25 parts, rhizoma nardostachyos 20 parts, gastrodia elata 12 parts and uncaria 10 parts by weight, and the sodium valproate is prepared into capsules, tablets, particles and other drug forms after pharmaceutical excipients such as starch or edible cellulose are added according to a common pharmacy method. A compound sodium valproate drug composition treats both principal and secondary aspect of epilepsia disease, is non-toxic harmless and free of side effect to human body, low in price, simple and convenient to manufacture, and has wide application prospect.
Owner:仁和堂药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com