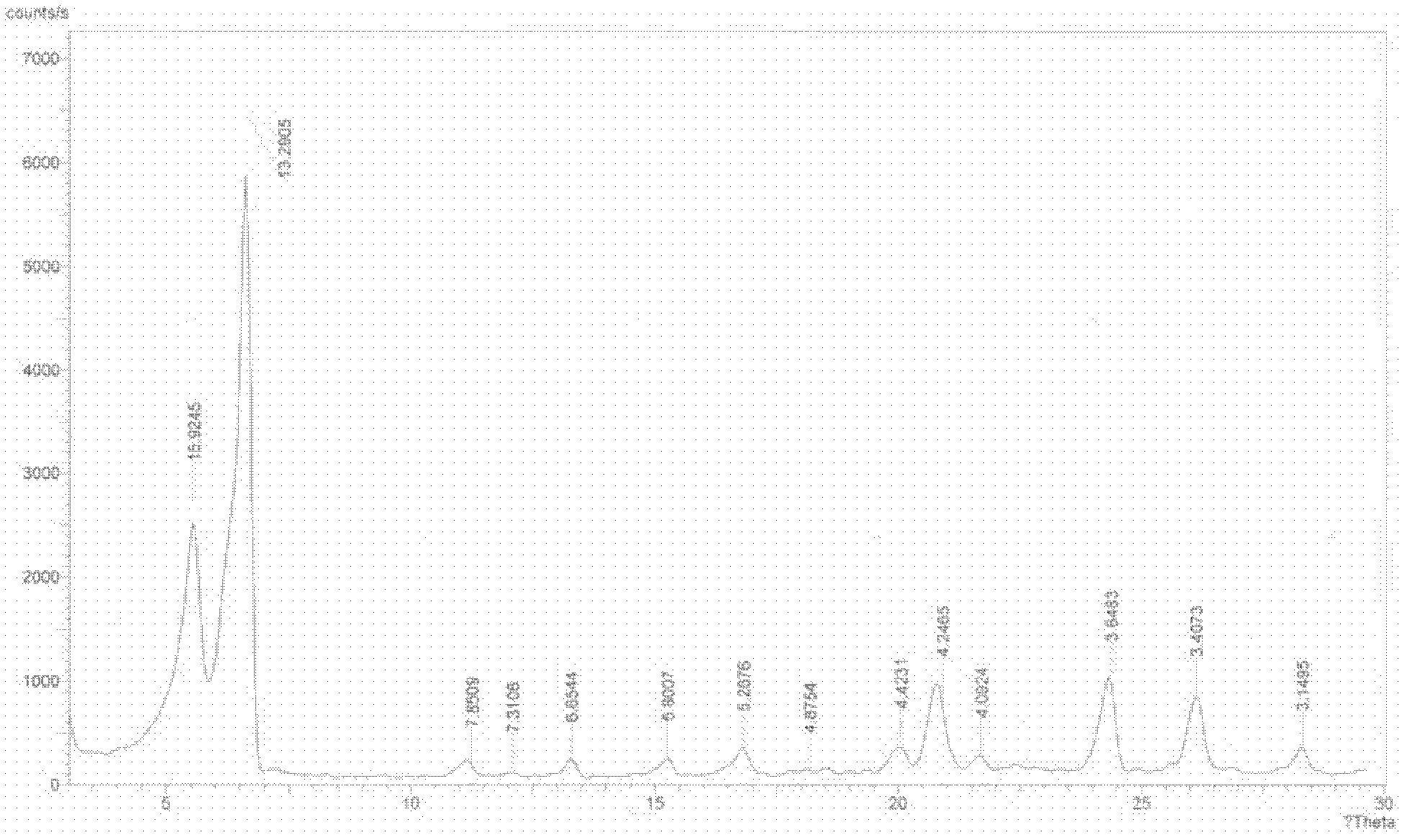Novel crystal form of sodium valproate, and preparation method and application thereof
A technology of sodium valproate, crystal form, applied in the direction of anhydride/acid/halide active ingredients, nervous system diseases, organic chemistry, etc., can solve the problems of reducing sodium valproate hygroscopicity, poor resolubility, etc. Dissolution time, low water content, pain-reducing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1 The preparation method of sodium valproate crystal form I of the present invention
[0029] Weigh 3.00kg of sodium valproate into the reaction kettle, then weigh 20.00kg of acetone into it, heat up to 60-90°C, and start to reflux, measure 0.05L of water into it, and then reflux for 25 minutes, suction filter, The filtrate was naturally cooled to below 25°C at room temperature, crystallized for 2 to 4 hours, filtered with suction, and the filter cake was washed twice with 3.00 kg of acetone. After fully draining the solvent, the filter cake was dried to obtain sodium valproate crystal form I.
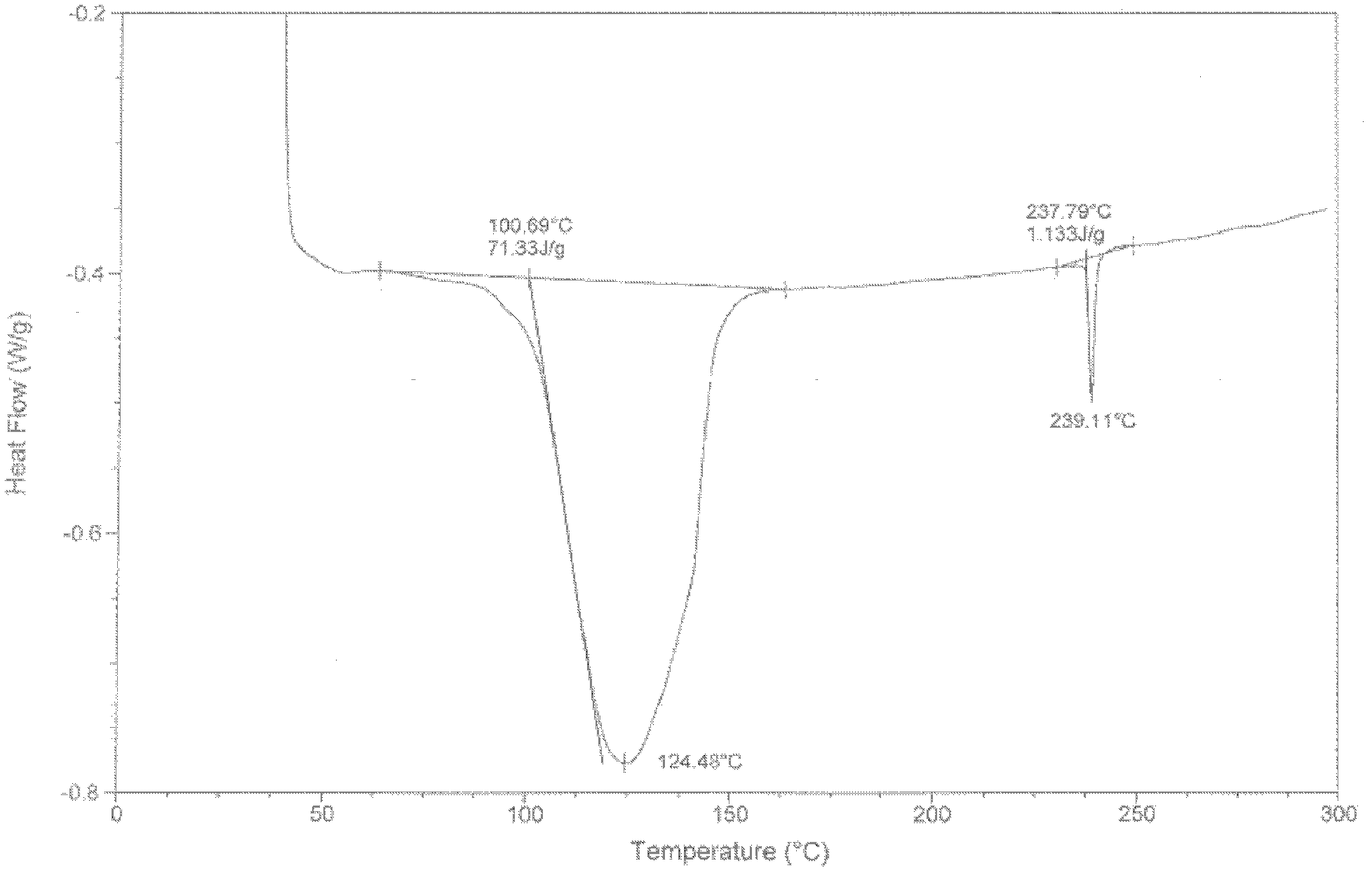
[0030] The P-XRD pattern of crystal form I is shown in figure 1 , see the DSC spectrum figure 2 .
[0031] Wherein, in the X-ray diffraction pattern, there are characteristic absorption peaks at (2θ)=5.54°, 6.64°, 20.90°, 24.38° and 26.13°±0.1° (also can be expressed as 5.54°±0.1°, 6.64°±0.1°, 20.90°±0.1°, 24.38°±0.1° and 26.13°±0.1°).
[0032] In the present inv...
Embodiment 2
[0033] Embodiment 2 The preparation method of pharmaceutical composition of the present invention
[0034] Under aseptic conditions, after the sodium valproate crystal form I was prepared by using Example 1, the crystal form I was put into a glass bottle and sealed to obtain the powder injection of sodium valproate crystal form I.
[0035] The beneficial effects of the present invention will be specifically described below through test examples.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com