Composition comprising prostaglandin derivatives and ophthalmic liquid formulation comprising the composition
A technology of prostaglandins and derivatives, applied in the field of medicine, can solve problems such as adverse reactions, hair loss, liver toxicity, etc., and achieve the effect of avoiding the use of co-solvents and improving safety characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
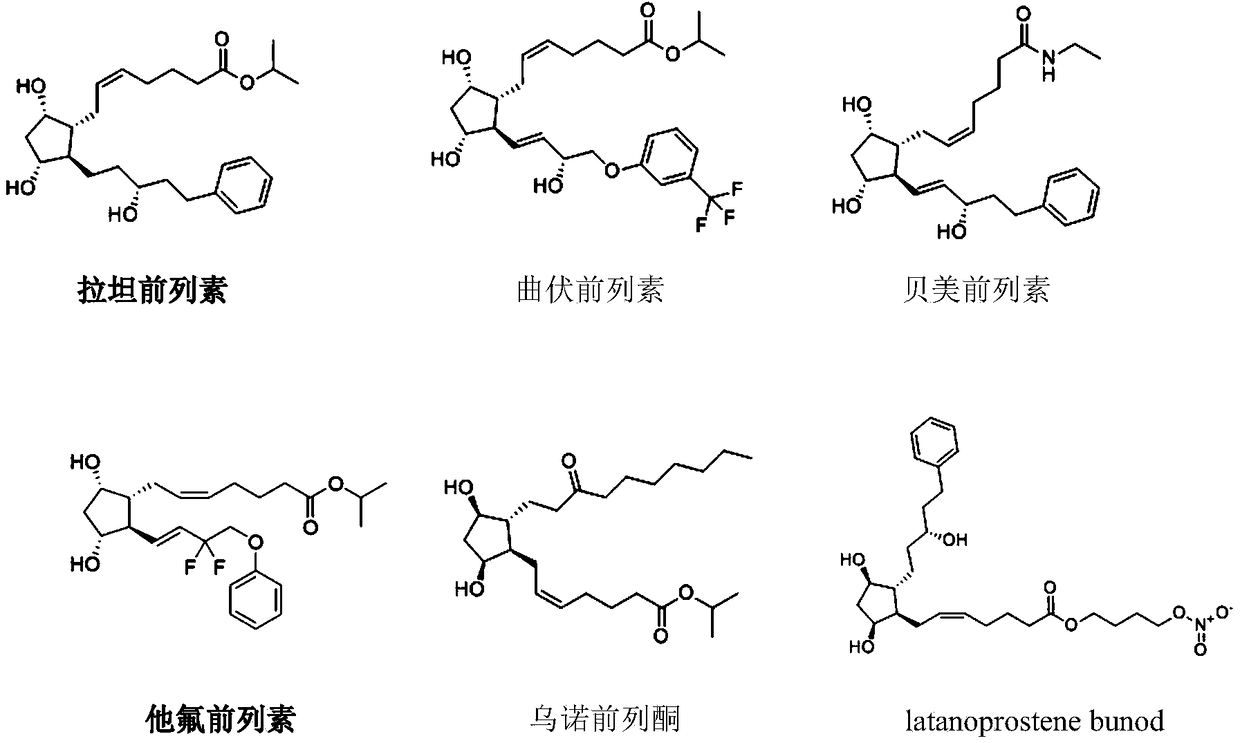
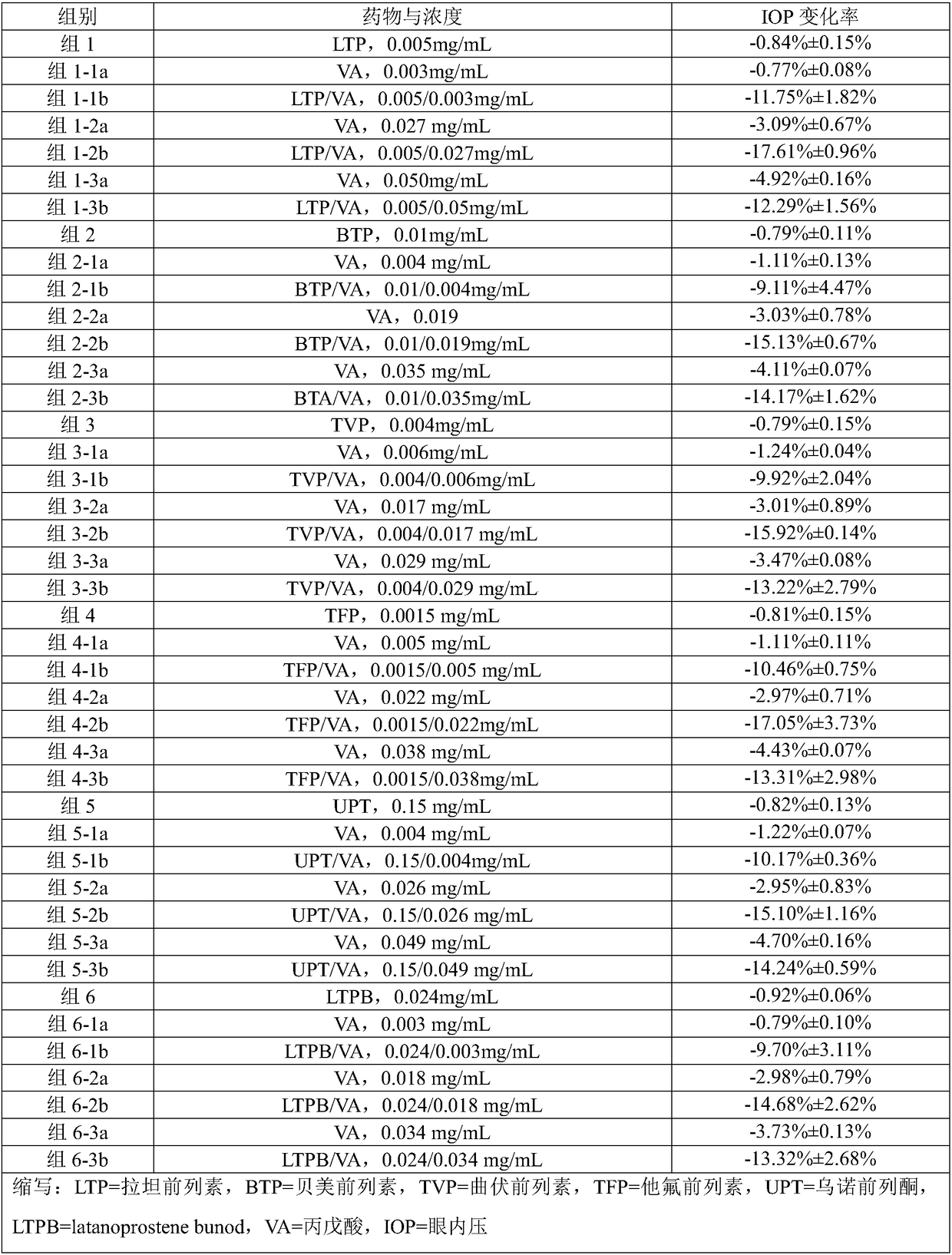
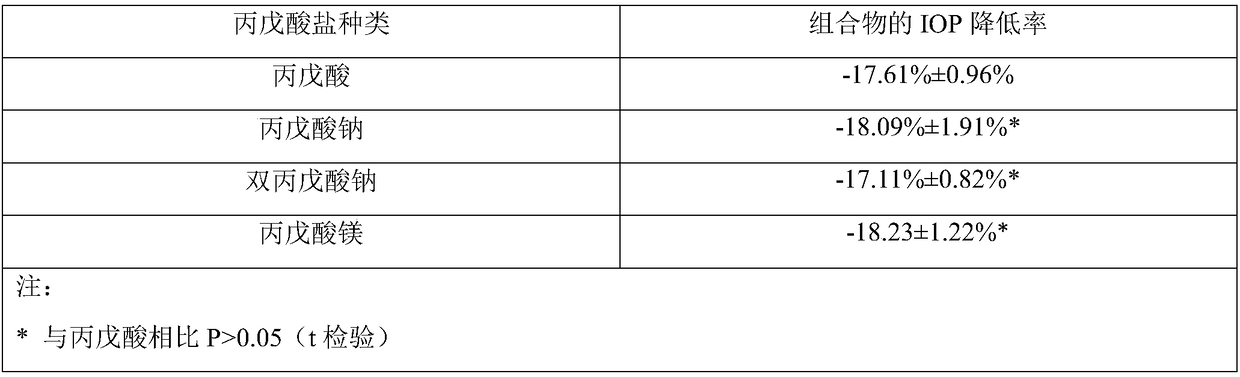
[0059] Example 1 Preparation of Ophthalmic Liquid Preparation Containing Latanoprost and Valproic Acid (1:16)
[0060] prescription
[0061]
[0062] Preparation
[0063] Mix and dissolve the prescribed amount of latanoprost, valproic acid, sodium chloride, edetate disodium, glycerin and benzalkonium chloride in 800mL water for injection, add the remaining amount of water for injection, sterilize, and pack , that is.
Embodiment 2
[0064] Example 2 Preparation of Ophthalmic Liquid Preparation Containing Bimatoprost and Sodium Valproate (1:6)
[0065] prescription
[0066]
[0067]
[0068] Preparation
[0069] Take the prescribed amount of travoprost, sodium valproate, sodium chloride, polyvinyl alcohol, benzalkonium chloride and carmellose sodium, mix and dissolve in 800mL water for injection, then add the remaining water for injection, and sterilize , sub-package, that is.
Embodiment 3
[0070] Example 3 Preparation of an ophthalmic liquid preparation comprising travoprost and divalproex sodium (1:15)
[0071] prescription
[0072]
[0073] Preparation
[0074] Take the prescribed amount of travoprost, divalproex sodium, sodium bisulfite, sodium chloride, glycerin and ethyl paraben, mix and dissolve in 800mL water for injection, then add the remaining amount of water for injection, sterilize, Subpackage, that is.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com