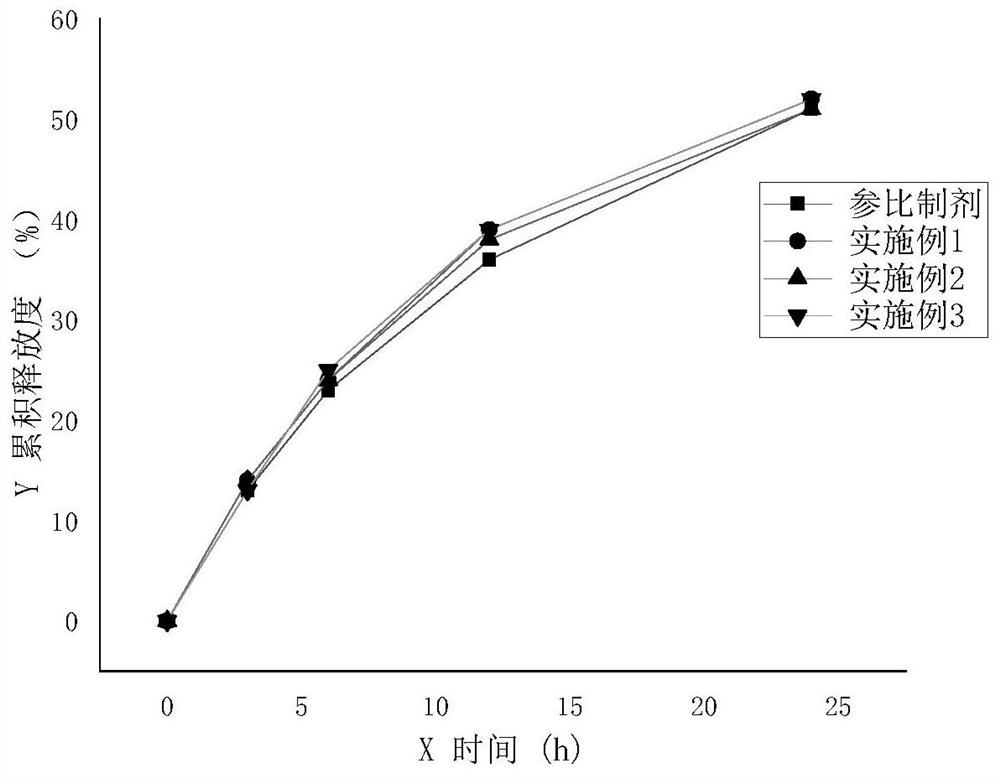
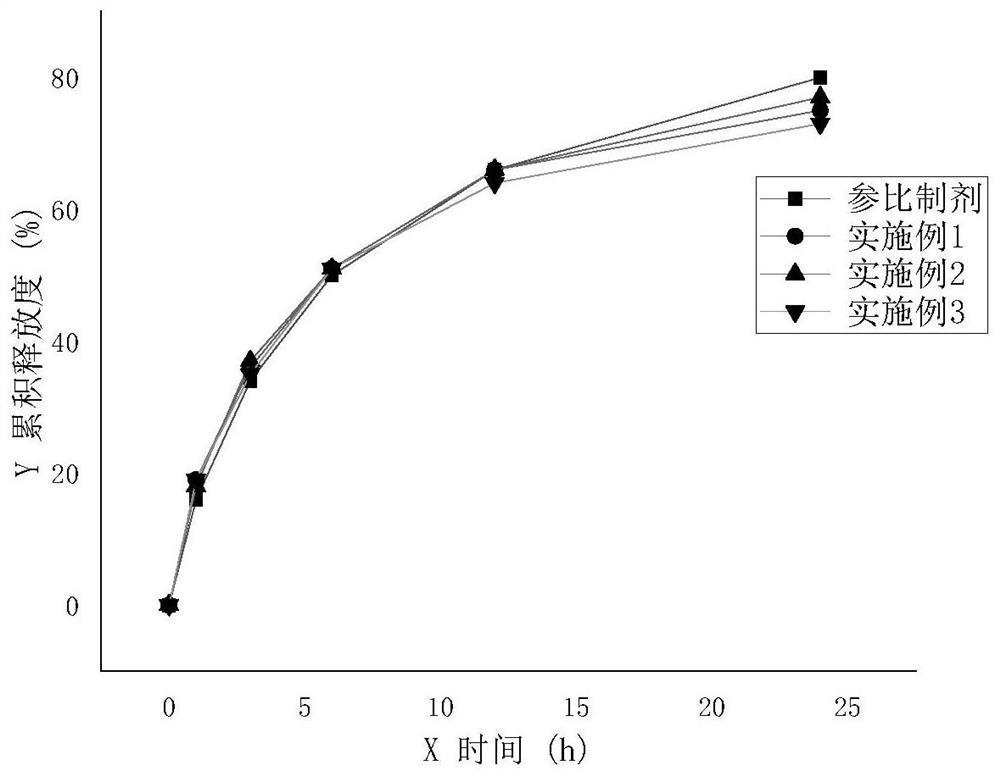
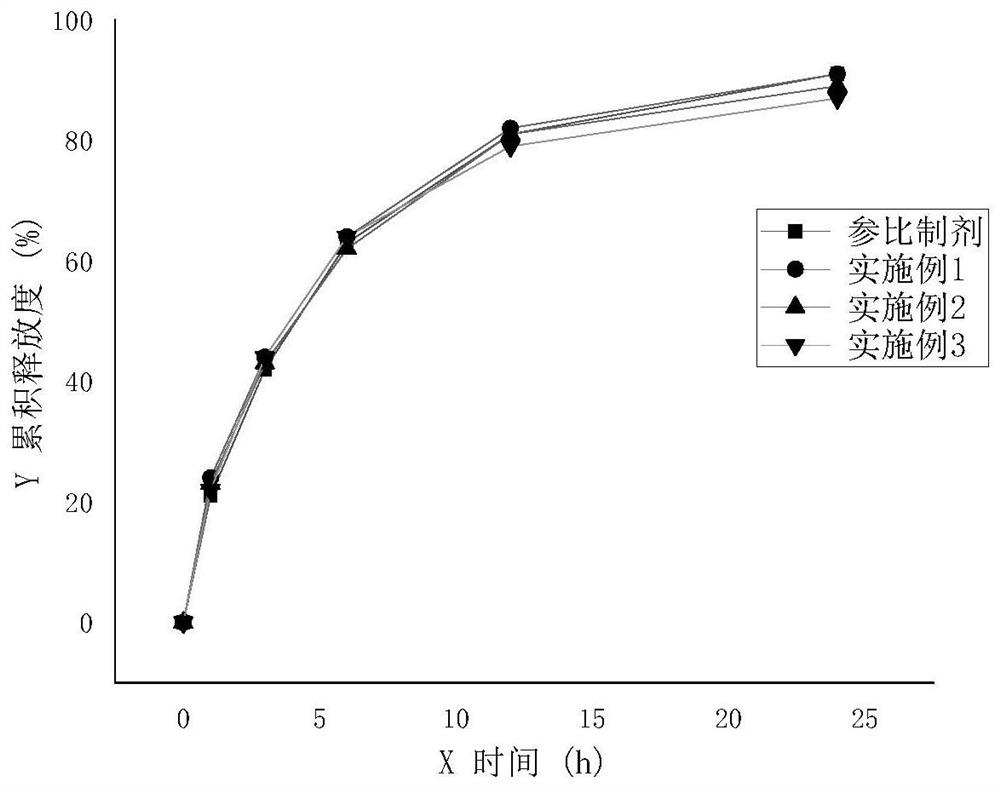
Preparation method of sodium valproate sustained release tablet
A technology of sodium valproate and sustained-release tablets, which is applied in the field of medicine, can solve the problems of easy spherical shape, low moisture content, slow particle growth, etc., and achieve the effect of overcoming the consequences of collapsed bed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0076] The preparation process of the original trituration agent is as follows:
[0077] The raw and auxiliary materials in the tablet core are simply mixed, and tabletable granules can be obtained without the need for reagents and drying. The granules are compressed and finally coated. The environment does not require dehumidification. By repeating the method and simply mixing the raw and auxiliary materials, the particles have poor fluidity, and due to the large amount of fine powder and the strong hygroscopicity of sodium valproate, the sticking is serious during the tableting process.
[0078] (5), fluidized bed granulator
[0079] The structure of the fluidized bed granulator is as follows: Figure 5 As shown, it includes an air treatment system 1 , a pot body 2 , a spray gun 3 , a filter 4 , an exhaust gas treatment system 5 and a humidifier 6 . In the air treatment system 1, the controlled temperature and humidity entering the fluidized bed can be monitored, and the ...
Embodiment 1
[0082] Example 1: In order to demonstrate that this technology can be used for scale-up production, 100,000 pieces of products are produced in this example.
[0083] A preparation method of sodium valproate sustained-release tablet, comprising the steps:
[0084] 1. Adsorption
[0085] Pour valproic acid into silica, the mass ratio of valproic acid to silica is 4:1, stir to solidify into powder, and pass the powder through an 80-mesh sieve to obtain valproic acid adsorbate.
[0086] 2. Mix
[0087] Pour the hypromellose, sodium valproate, valproic acid adsorbate, ethyl cellulose and sodium saccharin into the wet granulator, set stirring at 150 rpm, cut the knife at 2000 rpm, and mix for 5 minutes to prepare a premix .
[0088] 3. Granulation
[0089] Pour the premix into the fluidized bed pot, turn off the heating, set the inlet air humidity to 70±5%, and fluidize for 126 minutes to form wet granules.
[0090] Pour the premix directly into the fluidized bed pot, turn off ...
Embodiment 2
[0099] Example 2: Using the same recipe as Example 1
[0100] The preparation process is as follows:
[0101] 1. Adsorption
[0102] Pour valproic acid into silica, the mass ratio of valproic acid to silica is 5:1, stir to solidify into powder, and pass the powder through an 80-mesh sieve to obtain valproic acid adsorbate.
[0103] 2. Mix
[0104] Pour the hypromellose, sodium valproate, valproic acid adsorbate, ethyl cellulose and sodium saccharin into the wet granulator, set stirring at 150 rpm, cut the knife at 2000 rpm, and mix for 5 minutes to prepare a premix .
[0105] 3. Granulation
[0106] Pour the premix directly into the fluidized bed pot, turn off the heating, and set the air volume to 600m 3 / h, the bag shaking interval is 30s, the bag shaking time is 15s, and the inlet air humidity is 90±5%. Granulate for 30 minutes to form wet granules.
[0107] 4. Dry
[0108] Set the inlet air temperature to 70°C, set the inlet air humidity to 40%, and the air volume to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com