Irbesartan and hydrochlorothiazide tablet and preparation method thereof
A technology of chlorothiazide tablets and hydrochlorothiazide, which is applied in the direction of pill delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve problems such as poor fluidity and sticky punching, improve solubility, avoid sticky punching, and avoid content The effect of substandard uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
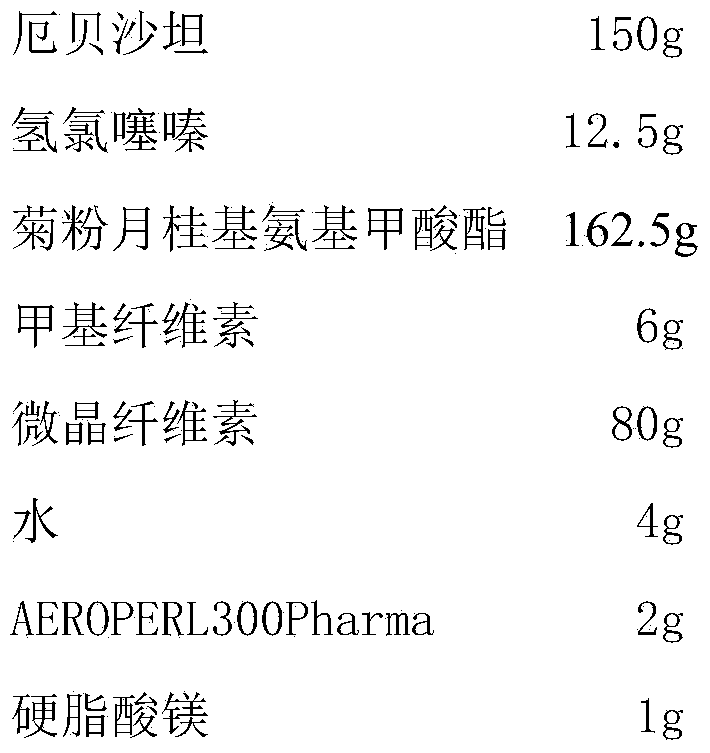
[0021] Embodiment 1 prepares the irbesartan hydrochlorothiazide tablet of the present invention
[0022] prescription:
[0023]
[0024] Preparation:
[0025] (1) Preparation of medicinal premix: Take inulin lauryl carbamate and heat it in a water bath at 80°C to make it molten, add irbesartan and hydrochlorothiazide, stir well, and put it in the refrigerator at -15°C to solidify. Pulverize, place in a vacuum drier at room temperature and dry to constant weight, pulverize and pass through an 80-mesh sieve to obtain a medicinal premix;
[0026] (2) Compressed tablets: Mix the medicinal premix with methylcellulose and microcrystalline cellulose under high shear for 1.5 minutes, spray water on the mixture for 3 minutes, add AEROPERL300Pharma and mix for 3 minutes to obtain dry granules. Finally, magnesium stearate was added and mixed for 30 seconds, and the tablet was obtained.
Embodiment 2
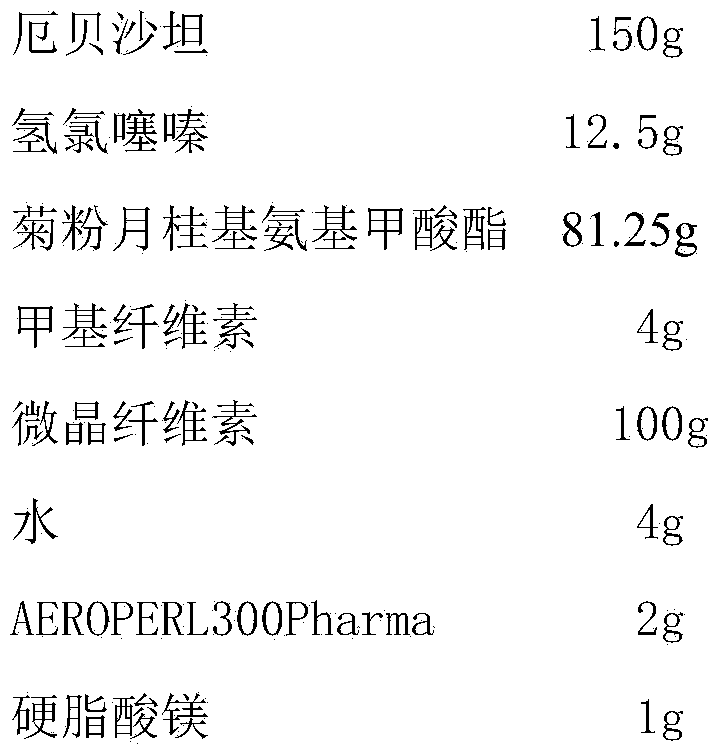
[0027] Embodiment 2 prepares the irbesartan hydrochlorothiazide tablet of the present invention
[0028] prescription:
[0029]
[0030] Preparation:
[0031] (1) Preparation of medicinal premix: Take inulin lauryl carbamate and heat it in a water bath at 80°C to make it molten, add irbesartan and hydrochlorothiazide, stir well, and put it in the refrigerator at -15°C to solidify. Pulverize, place in a vacuum drier at room temperature and dry to constant weight, pulverize and pass through an 80-mesh sieve to obtain a medicinal premix;
[0032] (2) Compressed tablets: Mix the medicinal premix with methylcellulose and microcrystalline cellulose under high shear for 1.5 minutes, spray water on the mixture for 3 minutes, add AEROPERL300Pharma and mix for 3 minutes to obtain dry granules. Finally, magnesium stearate was added and mixed for 30 seconds, and the tablet was obtained.
Embodiment 3
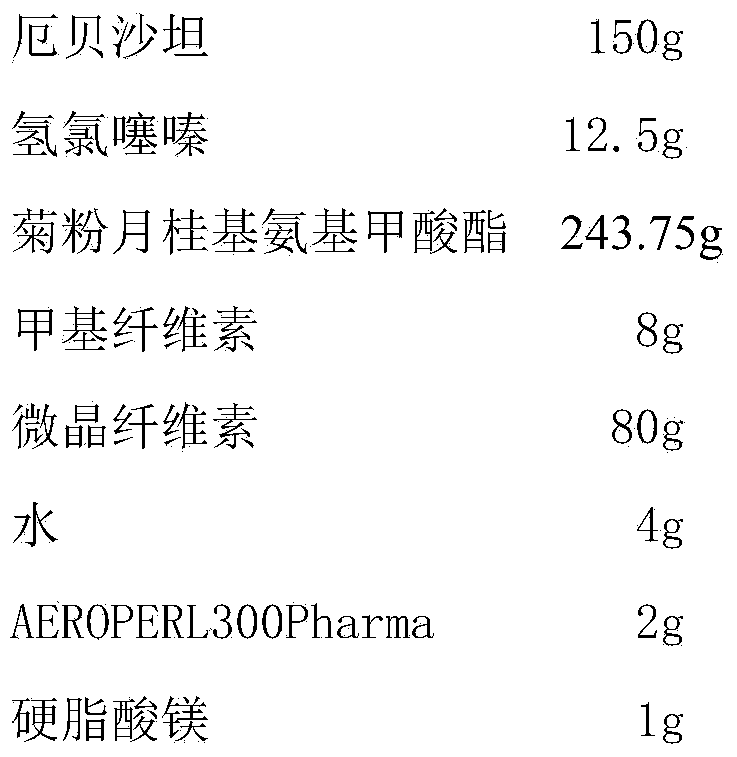
[0033] Embodiment 3 prepares irbesartan hydrochlorothiazide tablet of the present invention
[0034] prescription:
[0035]
[0036] Preparation:
[0037] (1) Preparation of medicinal premix: Take inulin lauryl carbamate and heat it in a water bath at 80°C to make it molten, add irbesartan and hydrochlorothiazide, stir well, and put it in the refrigerator at -15°C to solidify. Pulverize, place in a vacuum drier at room temperature and dry to constant weight, pulverize and pass through an 80-mesh sieve to obtain a medicinal premix;
[0038] (2) Compressed tablets: Mix the medicinal premix with methylcellulose and microcrystalline cellulose under high shear for 1.5 minutes, spray water on the mixture for 3 minutes, add AEROPERL300Pharma and mix for 3 minutes to obtain dry granules. Finally, magnesium stearate was added and mixed for 30 seconds, and the tablet was obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com