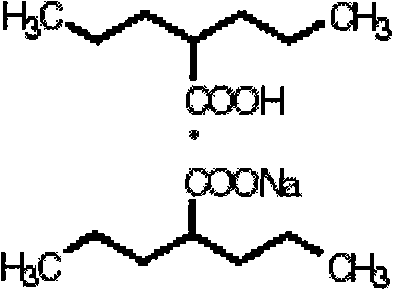
Double sodium valproate orally disintegrating tablets and preparation method thereof
A technology of sodium divalproex, orally disintegrating tablets, applied in the direction of anhydride/acid/halide active ingredients, nervous system diseases, pill delivery, etc. Headache, high bioavailability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The orally disintegrating tablet of divalproex sodium comprises main ingredients and auxiliary materials, and is characterized in that it is formulated according to the following weight percentages: 10% of main ingredients and 90% of auxiliary materials.
[0036] Divalproex Sodium 25g (10%)
[0037] Microcrystalline Cellulose 37.5g (20%)
[0038] Mannitol 92.5g (37%)
[0039] Lactose 55g (22%)
[0040] Polyacrylic resin II 12.5g (5%)
[0041] Cross-linked polyvinylpyrrolidone 7.5g (3%)
[0042] Cyclamate 2.5g(1%)
[0043] Menthol 2.5(1%)
[0044] Micronized silica gel 2.5(1%)
[0045]
[0046] A total of 1000 tablets were produced
[0047] Among them, lactose and mannitol are fillers, microcrystalline cellulose and cross-linked polyvinylpyrrolidone are disintegrants, polyacrylic acid resin II, cyclamate and menthol are flavoring agents, and micronized silica gel is a glidant.
[0048] The preparation method of divalpro...
Embodiment 2
[0058]The orally disintegrating tablet of divalproex sodium comprises main ingredients and auxiliary materials, and is characterized in that it is formulated according to the following weight percentages: 20% of main ingredients and 80% of auxiliary materials.
[0059] Divalproex Sodium 50g (20%)
[0060] Microcrystalline Cellulose 32.5g (13%)
[0061] Mannitol 110g (38%)
[0062] Lactose 45g (15%)
[0063] Polyacrylic resin II 12.5g (5%)
[0064] Cross-linked polyvinylpyrrolidone 12.5g (5%)
[0065] Cyclamate 2.0g(1%)
[0066] Menthol 2.5(1%)
[0067] Micronized silica gel 5.0(2%)
[0068]
[0069] A total of 1000 tablets were produced
[0070] Among them, lactose and mannitol are fillers, microcrystalline cellulose and cross-linked polyvinylpyrrolidone are disintegrants, polyacrylic acid resin II, cyclamate and menthol are flavoring agents, and micronized silica gel is a glidant.
[0071] The preparation method of divalproex sod...
Embodiment 3
[0081] The orally disintegrating tablet of divalproex sodium comprises main ingredients and auxiliary materials, and is characterized in that it is formulated according to the following weight percentages: 30% of main ingredients and 70% of auxiliary materials.
[0082] Divalproex Sodium 75g (30%)
[0083] Microcrystalline Cellulose 25g (10%)
[0084] Mannitol 100g (40%)
[0085] Lactose 12.5g (5%)
[0086] Polyacrylic resin II 12.5g (5%)
[0087] Cross-linked polyvinylpyrrolidone 12.5g (5%)
[0088] Cyclamate 2.0g(1%)
[0089] Menthol 2.5(1%)
[0090] Micronized silica gel 7.5(3%)
[0091]
[0092] A total of 1000 tablets were produced
[0093] Among them, lactose and mannitol are fillers, microcrystalline cellulose and cross-linked polyvinylpyrrolidone are disintegrants, polyacrylic acid resin II, cyclamate and menthol are flavoring agents, and micronized silica gel is a glidant.
[0094] The preparation method of divalproex sod...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com