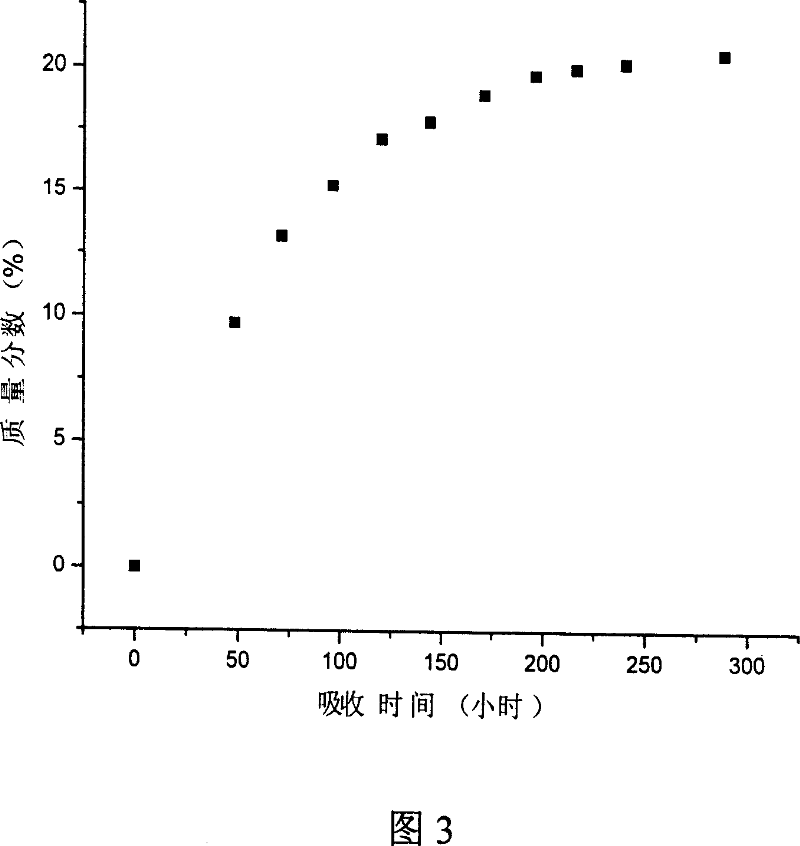
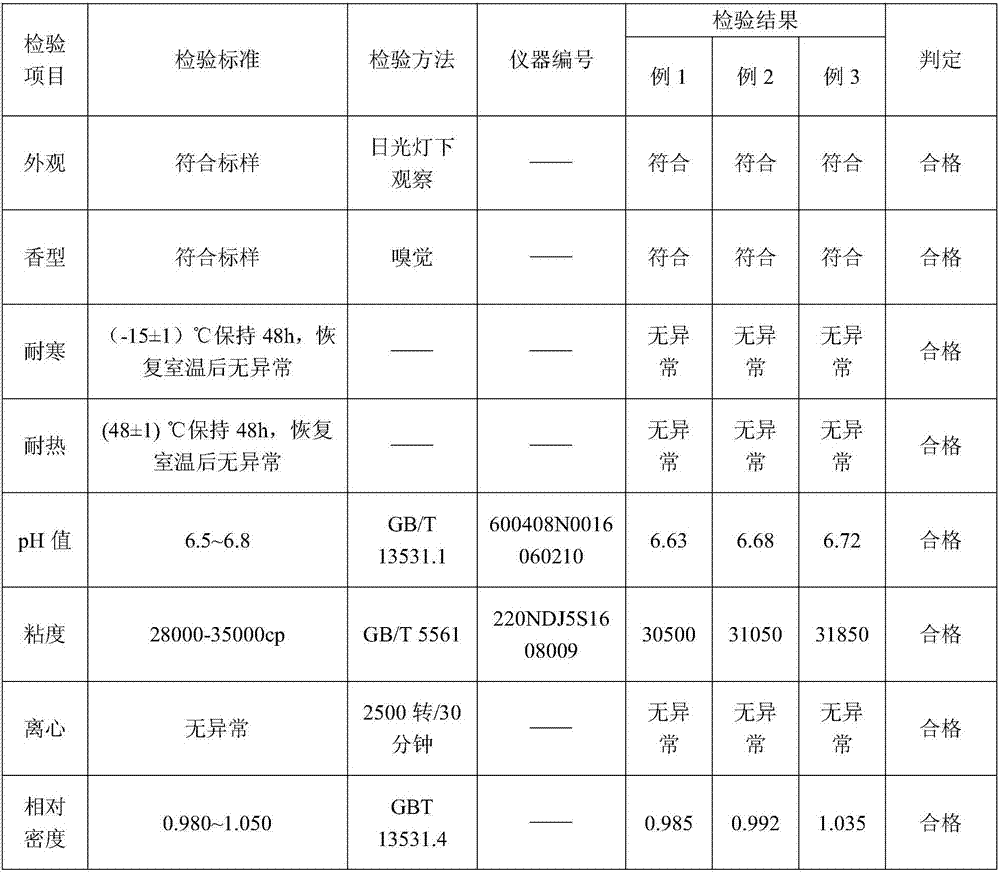
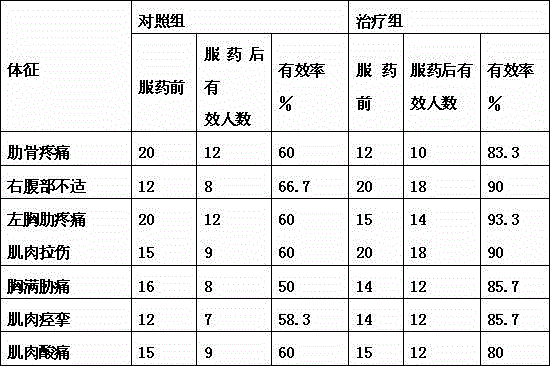
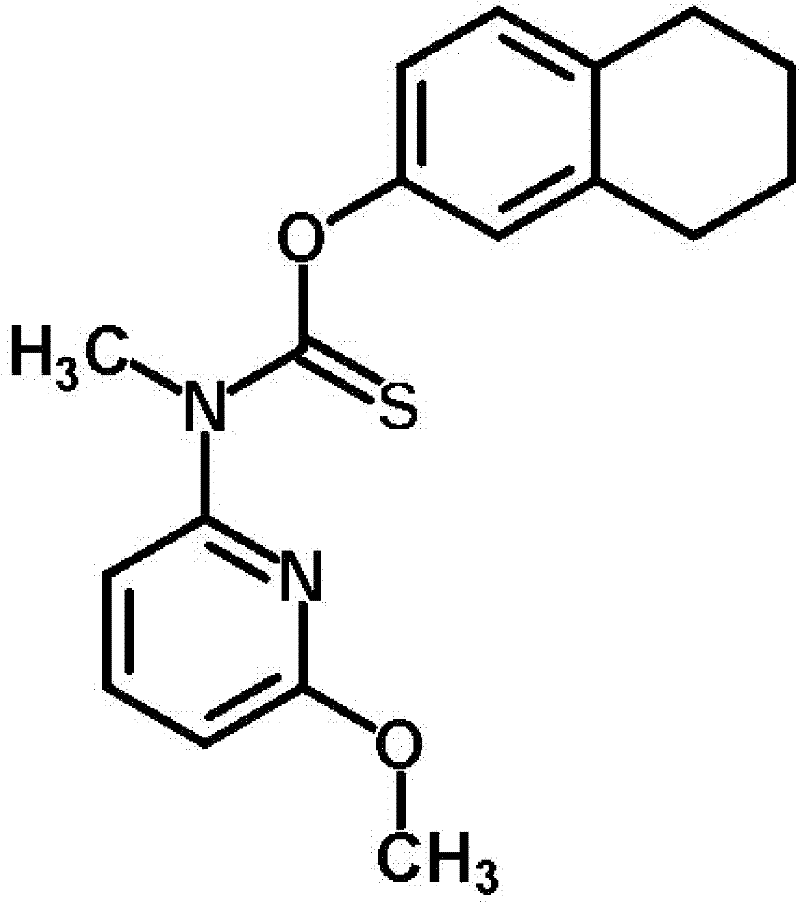
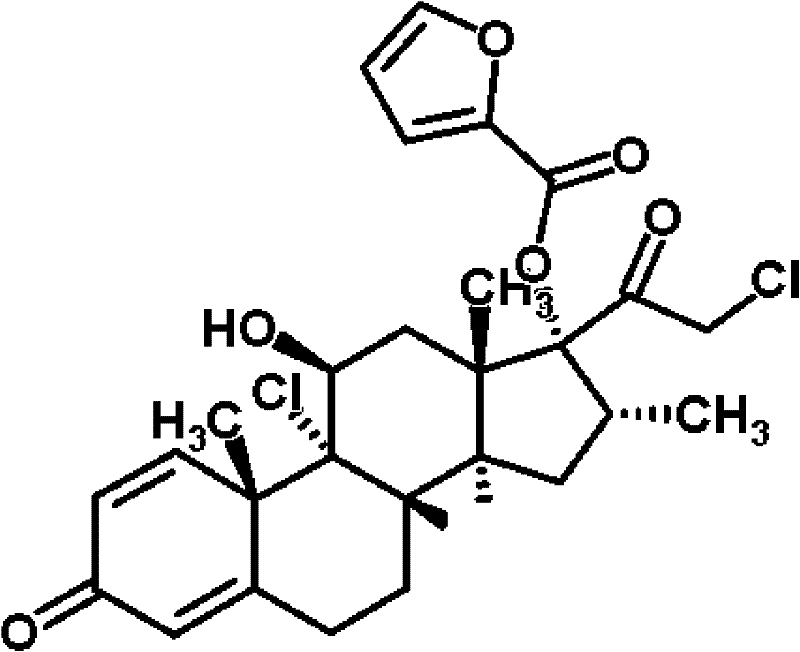
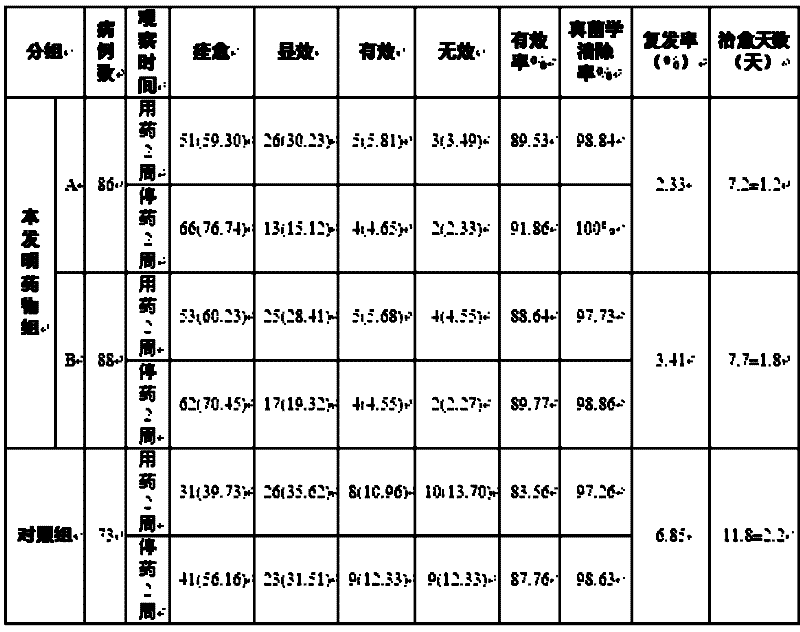
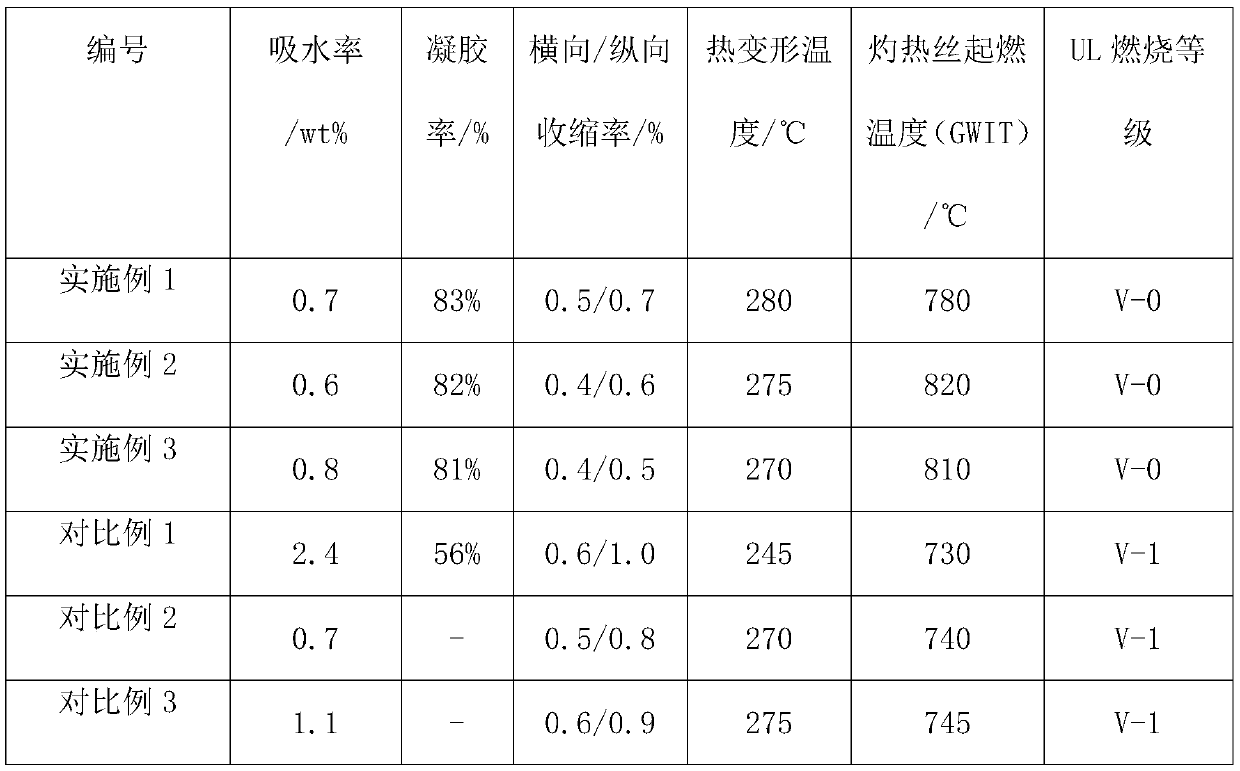
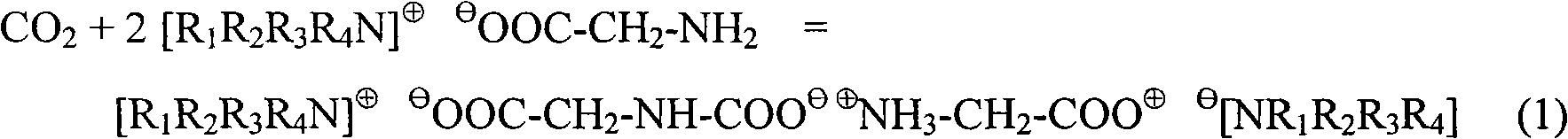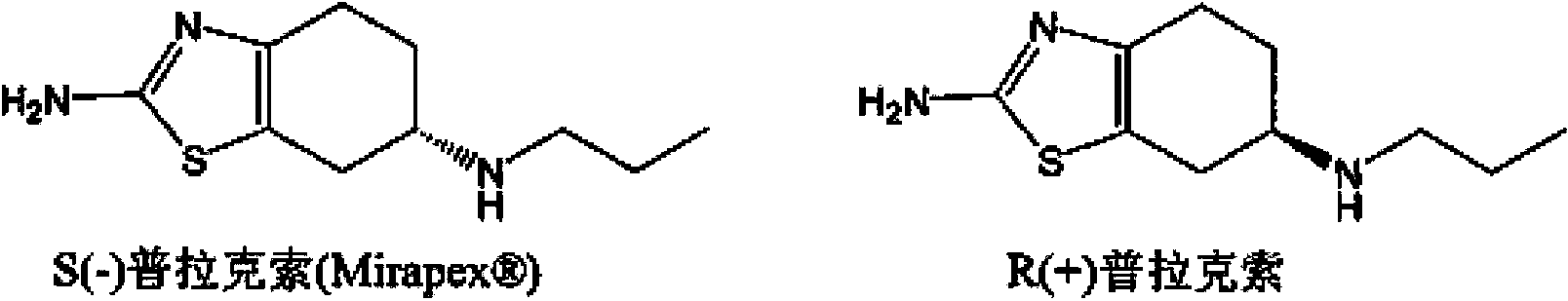Patents
Literature
68results about How to "Reduce absorption rate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
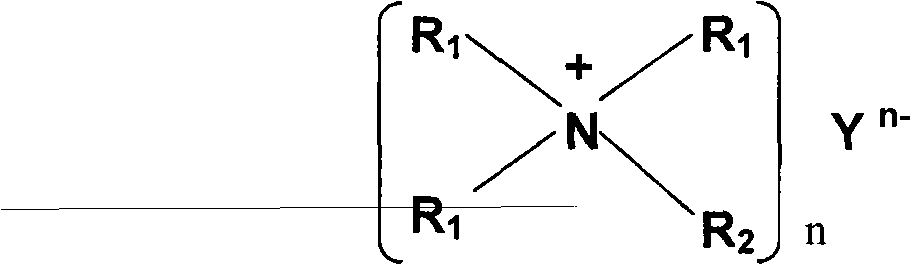
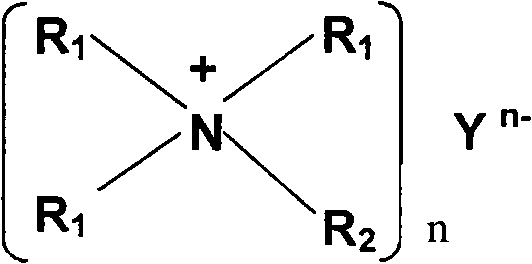
Special MDEA formula solution activated by functional ion liquid for CO2 gas absorption separation
InactiveCN101804292AReduce lossOvercome the disadvantage of high energy consumptionDispersed particle separationBy chemical separationSulfolanePoly(ethylene glycol) dimethyl ether
The invention relates to a special N-methyldiethanolamine formula solution activated by ion liquid for CO2 gas absorption separation, which consists of the following ingredients in mass percent: 35 to 50 percent of N-methyldiethanolamine, 5 to 20 percent of low-viscosity kalescent functional ion liquid, 15 to 30 percent of dimethyl ether of polyethlene glycol and / or sulfolane and 15 to 30 percent of water, wherein cations of the low-viscosity kalescent functional ion liquid are tetraalkylammonium ions, and anions of the low-viscosity kalescent functional ion liquid are amino acid radicals or organic carboxylate anions. The formula solution of the invention has the advantages that the high mass transfer performance of the absorption-desorption process is improved, the material consumption in the use process is low, the defect of high energy consumption because a large amount of water vapor is brought away during the absorbing agent regeneration, and the invention belongs to an energy-saving formula with high green degree. The regeneration temperature of the solution is lower than that of the traditional absorbing liquid, the grade of a heat source required to be provided in the regeneration process is reduced, energy sources can be saved, the stability of the absorbing agent solution in the operation is high, the consumption of each absorption-desorption circulation is low, and in addition, the cost is low.
Owner:NANJING UNIV
Multifunctional microemlusion gel preparation and preparation process thereof
InactiveCN103655459AImprove skin penetrationImprove performanceAntimycoticsAntipyreticIndometacinActive agent
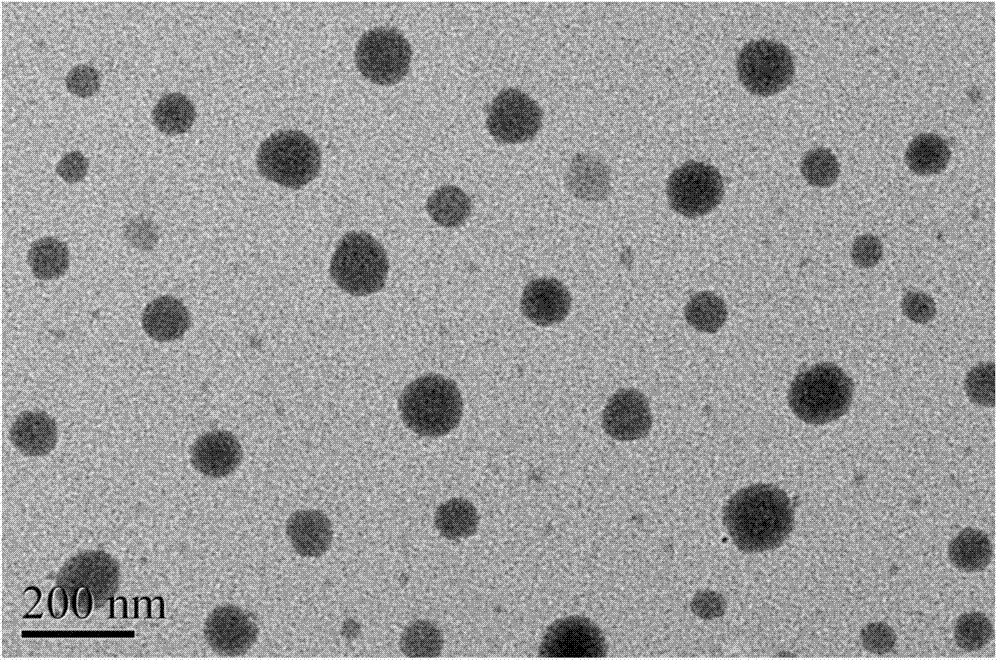
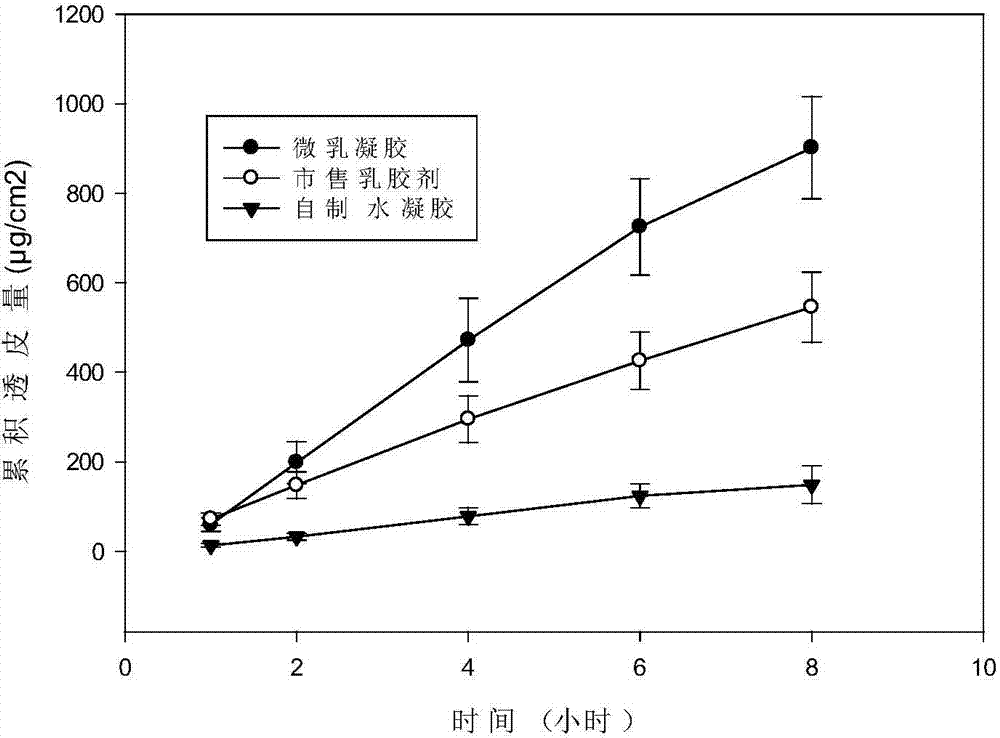
The invention discloses a multifunctional microemlusion gel preparation and a preparation process thereof, and belongs to the technical field of medicines. The preparation mainly comprises bulk pharmaceutical chemicals (such as non-steroidal anti-inflammatory drugs-diclofenac sodium, ibuprofen, indometacin, antifungal drugs-ornidazole, antiviral drugs-ganciclovir, hormone drugs-dexamethasone, local anesthesia drugs-lidocaine and irritants-menthol), a cationic polymer and a microemlusion, can further comprise gel or a thickener, and can be used for transdermal drug delivery and local drug delivery. The preparation process is simple, convenient, good in stability and pollution-free. Compared with existing cream and gel, the preparation has the advantages that a novel action mechanism is adopted, the accumulative penetration amount of unit area of drugs is remarkably increased, a certain slow-release effect is achieved, and the drug delivery frequency and the drug delivery amount can be reduced; a chemical penetration enhancer and a conventional preservative are not added, a certain bacterial inhibition effect is achieved, the skin irritation is avoided, and the use safety of the drugs is improved.
Owner:CHINA PHARM UNIV
Clonidine hydrochloride sustained release tablets and preparation method thereof
InactiveCN104352473AImprove stabilityDefinite curative effectOrganic active ingredientsPharmaceutical product form changeExtended release tabletsPlastic packaging
The invention provides clonidine hydrochloride sustained release tablets. The clonidine hydrochloride sustained release tablets are prepared from 0.2 part of clonidine hydrochloride, 70-90 parts of sustained release skeleton material and 10-20 parts of lubricating agent by weight. A preparation method of the clonidine hydrochloride sustained release tablets comprises the steps of material preparing, blending, granulating, blending, tabletting and aluminium-plastic packaging. The clonidine hydrochloride sustained release tablets and the preparation method have the beneficial effects that the clonidine hydrochloride sustained release tablets have good stability and definite curative effects and can be used for effectively treating hypertension; the novel sustained release preparations are adopted; sustained release refers to reducing the medicine release rates of medicines from the dosage forms and reducing the absorption rates of the medicines into bodies, thus achieving more stable treatment effects; compared with oral liquids, the clonidine hydrochloride sustained release tablets have the advantages of good medicine stability, convenience in packaging, transportation and storage, and the like; the preparation method is simple and practicable and is suitable for industrial production.
Owner:HARBIN SHENGJI PHARMA
Method and device for measuring absorbing capacity of organic solvent volatile gas in ion liquid
InactiveCN101038249AFacilitate desorptionEasy to desorbWeighing by absorbing componentIonSaturable absorption
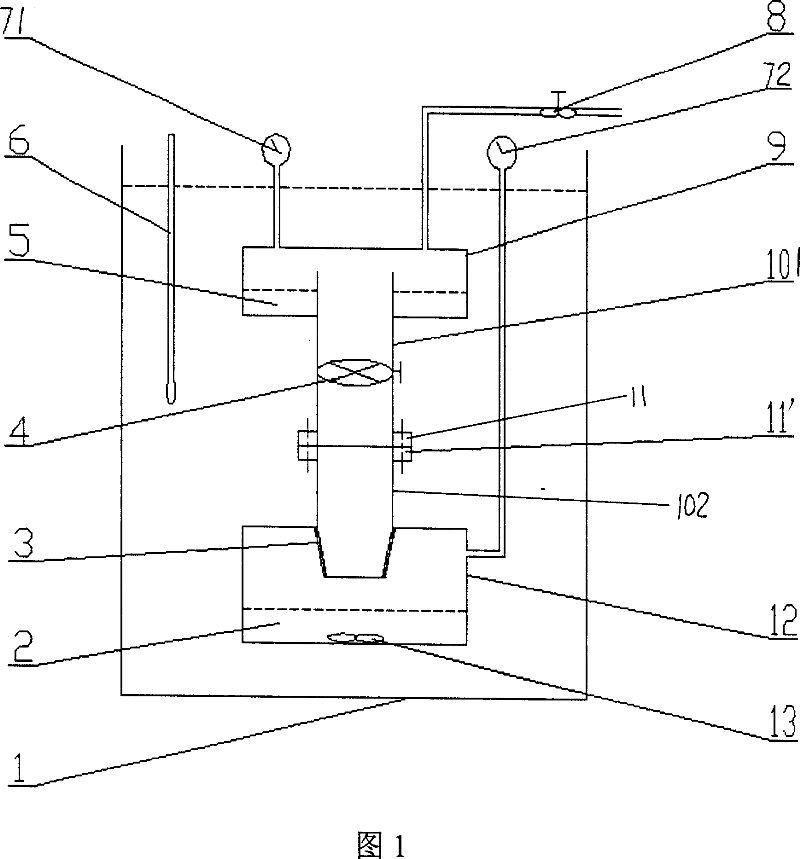
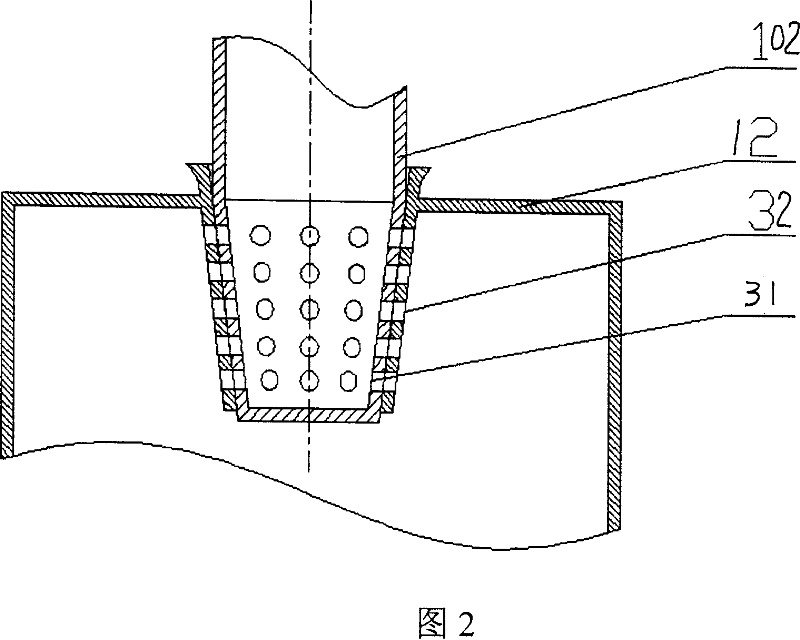
The present invention relates to a method and apparatus used for measuring the absorbing amount of an organic solvent volatile gas in an ionic liquid. Said apparatus comprises an evaporator used for a natural evaporation of an organic solvent and an absorber used for adsorbing natural volatile gases of a variety of organic solvents utilizing an ionic liquid as an absorbing agent to achieve the intention that an ionic liquid is used as an absorbing agent to remove organic solvents in industrial waste gas. A weighing method is utilized to perform detections, then the transient absorbing capacity and saturated absorbing capacity of an organic volatile gas in an ionic liquid are got through the mass difference after and before the absorption by the absorbing agent. The channel valve adopts a rotary switch type valve, which is capable of both increasing the flux and decreasing the valve weight at the same time to ensure the weighing precision. The absorber is equipped with a magnetic stirring therein and the adsorbing process is a deep absorption.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Water-in-oil-in-water CC cream and preparation method thereof
InactiveCN107157782AReduce absorption rateGood moisturizing and repairing effectCosmetic preparationsToilet preparationsWater in oilOil and grease
The invention discloses water-in-oil-in-water CC cream and belongs to the technical field of cosmetics. The water-in-oil-in-water CC cream comprises, by weight, 0.1 to 10 parts of an emulsifier, 5 to20 parts of skin soothing oil, 5 to 20 parts of a sun-screening agent, 1 to 5 parts of a thickener, 1 to 5 parts of a stabilizer, 5 to 20 parts of a humectant, 1 to 10 parts of plant extract, 1 to 10parts of a colorant and 1 to 5 parts of water. The water-in-oil-in-water CC cream has effects of oil-in-water CC cream, such as covering spots or marks on the skin, adjusting skin color, preventing sunscreen and preventing water and swear for a long time, and also has thin and moist feeling of water-in-oil CC cream.
Owner:DOCTOR PLANT GUANGDONG BIOTECHNOLOGY CO LTD
Composite material containing microelement for hard tissue repair and reconstruction and preparation method thereof
The invention relates to a composite material containing microelement for hard tissue repair and reconstruction and a preparation method thereof. The composite material further contains at least one of three microelements of strontium, zinc and copper acceptable in human body with the calcium mole ratio of 0.01-0.5% in ceramic component of calcium phosphate in the ceramic component containing the calcium phosphate and the substrate material of multi-amino acid polymer, wherein the mass ratio of the ceramic component of calcium phosphate is 30-65%, and the balance of multi-amino acid polymer polymerized by epsilon-aminocaproic acid and other amino acids. The process for preparing the calcium phosphate ceramic comprises the following steps of: adding microelement, then mixing the amino acid components evenly, removing water of various forms in the mixture at a temperature lower than or equal to 200 DEG C under the protection of inert gas, then composing in situ polymerization under the condition of 210-250 DEG C and pH of 6.5-7.5. The composite material is a bionic biomedical and tissue engineering material which has controllable degradation speed, good bioactivity and compatibility and overcomes deficiencies and problems of similar repair materials at present.
Owner:SICHUAN GUONA TECH
Fluvoxamine maleate sustained release tablets and preparation method thereof
InactiveCN104352471ADefinite curative effectLittle side effectsNervous disorderPharmaceutical product form changeCompulsive disordersPlastic packaging
The invention provides fluvoxamine maleate sustained release tablets. The fluvoxamine maleate sustained release tablets are prepared from 50 parts of fluvoxamine maleate, 50-150 parts of sustained release skeleton material and 5-25 parts of lubricating agent by weight. A preparation method of the fluvoxamine maleate sustained release tablets comprises the steps of material preparing, blending, granulating, blending, tabletting and aluminium-plastic packaging. The fluvoxamine maleate sustained release tablets and the preparation method have the beneficial effects that the fluvoxamine maleate sustained release tablets are used for treating depression and associated symptoms as well as symptoms of obsessive-compulsive disorder and have definite curative effects and small side effects; the novel sustained release preparations are adopted; sustained release refers to reducing the medicine release rates of medicines from the dosage forms and reducing the absorption rates of the medicines into bodies, thus achieving more stable treatment effects; compared with oral liquids, the fluvoxamine maleate sustained release tablets have the advantages of good medicine stability, convenience in packaging, transportation and storage, and the like; the preparation method is simple and practicable and is suitable for industrial production.
Owner:HARBIN SHENGJI PHARMA
Attachment for an electronic communications device
InactiveUS20160006473A1Reduce exposureReduce absorption ratePrinted circuit assemblingAntenna supports/mountingsElectronic communicationEngineering
An attachment for an electronic communications device including a conducting element that is coated on one side with layers of material and securely affixed to a non-conducting substrate such that the overall dimensions and thickness of the attachment are sufficiently small that it may be attached to a surface of an electronic communications device whilst allowing the use of any protective casing preferred by the user.
Owner:CLASSIC PROMOTIONS
Cyclobenzaprine hydrochloride sustained release tablets and preparation method thereof
InactiveCN104352474AReduce absorption rateImprove stabilityOrganic active ingredientsMuscular disorderMuscle spasmTreatment pain
The invention provides cyclobenzaprine hydrochloride sustained release tablets. The cyclobenzaprine hydrochloride sustained release tablets are prepared from 15 parts of cyclobenzaprine hydrochloride, 75-80 parts of sustained release skeleton material and 5-10 parts of lubricating agent by weight. A preparation method of the cyclobenzaprine hydrochloride sustained release tablets comprises the steps of material preparing, blending, granulating, blending, tabletting and aluminium-plastic packaging. The cyclobenzaprine hydrochloride sustained release tablets are adjuvant medicines for treating painful local muscle spasm and have the beneficial effects that the novel sustained release preparations are adopted; sustained release refers to reducing the medicine release rates of medicines from the dosage forms and reducing the absorption rates of the medicines into bodies, thus achieving more stable treatment effects; compared with oral liquids, the cyclobenzaprine hydrochloride sustained release tablets have the advantages of good medicine stability, convenience in packaging, transportation and storage, and the like. The preparation method is simple and practicable and is suitable for industrial production.
Owner:HARBIN SHENGJI PHARMA
Liranaftate and mometasone furoate containing locally applied compound pharmaceutical composition
ActiveCN102379879ASlowed transdermal absorption rateReduce absorption rateAntimycoticsAntisepticsArteriolar VasoconstrictionAntifungal drugs
The invention relates to the technical field of antimycotic medicaments, specifically to a liranaftate and mometasone furoate containing locally applied compound pharmaceutical composition, which contains one or more acceptable auxiliary materials for local application. The invention is characterized in that the weight ratio of liranaftate to mometasone furoate is 1:80-80:1; the balance contains matrix auxiliary materials; The pharmaceutical composition can be emulsifiable paste or ointment, gelata, a solution or an emulsion or a suspension, a coating agent, aerosol or a spraying agent or spray-film, a foaming agent and patch. With the application of the liranaftate and mometasone furoate compound pharmaceutical composition, as mometasone furoate has a vasoconstriction effect, the transdermal absorption rate of liranaftate slows down, local skin tissue concentration of liranaftate is increased, the medicament is minimized to penetrate through the skin into blood, and local skin concentration of the antimycotic medicament is maintained for a longer time.
Owner:南昌百济制药有限公司
Synergist for assisting wet-process desulphurization of thermal power plant, and application method of synergist
InactiveCN103432894AReduce surface tensionIncrease the speed of diffusionDispersed particle separationOrganic acidGas to liquids
The invention discloses a synergist for assisting wet-process desulphurization of a thermal power plant. The synergist comprises the following components in percentage by mass: 55%-60% of organic acid, 15%-20% of organic acid salt, 10%-15% of oxidized catalyst, and 10%-15% of chelating dispersing agent. The acidity of the organic acid is stronger than that of carbonic acid and weaker than that of sulphurous acid, and the organic acid salt is used for providing organic acid groups. The synergist can reduce the surface tension between gas and liquid and improve the dispersion speed of gas to liquid; the concentration of calcium ions in the liquid solution can be increased, the crystallization speed of sulfate ions can be accelerated, and the desulfurization efficiency of the system and the utilization rate of lime stone can be improved; in addition, the characteristics of calcium sulfate crystals can be changed, so that muddy crystals can be produced, the crystals can be prevented from being deposited at parts, such as a spray nozzle.
Owner:杨子江
Clonidine hydrochloride sustained release pellets
InactiveCN104352447AReduce absorption rateStable absorptionOrganic active ingredientsPharmaceutical non-active ingredientsSustained release pelletsAdhesive
The invention provides clonidine hydrochloride sustained release pellets. The clonidine hydrochloride sustained release pellets comprise medicine-containing pellets and enteric coating layers, wherein the medicine-containing pellets are coated by the enteric coating layers; the medicine-containing pellets comprise 0.1mg of clonidine hydrochloride, 180mg of hollow pellet cores and 10mg of adhesive; the enteric coating layers comprise 45-225mg of Eudragit NE30D and 7-68mg of talcum powder. A preparation method of the clonidine hydrochloride sustained release pellets comprises the following processes: 1. material preparation; 2. pellet preparation; 3. preparation of an enteric coating agent; 4. coating; 5. filling; 6. aluminium-plastic packaging and preparation of finished products. The sustained release pellets with clonidine hydrochloride as an active ingredient are mainly used for treating hypertension and have the beneficial effects that as the two kinds of advanced technologies, namely novel sustained release preparations and pellet preparations, are adopted, the clonidine hydrochloride sustained release pellets have stable treatment effects and higher bioavailability and have the advantages of good medicine stability, convenience in packaging, transportation and storage, and the like. The preparation method is simple and practicable and is suitable for industrial production.
Owner:HARBIN SHENGJI PHARMA
Tetrahydrobenzothiazole derivative and preparation method thereof
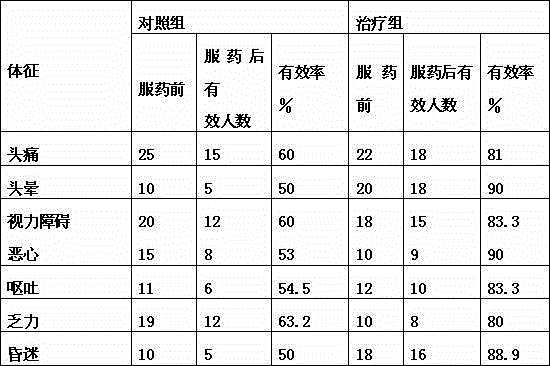
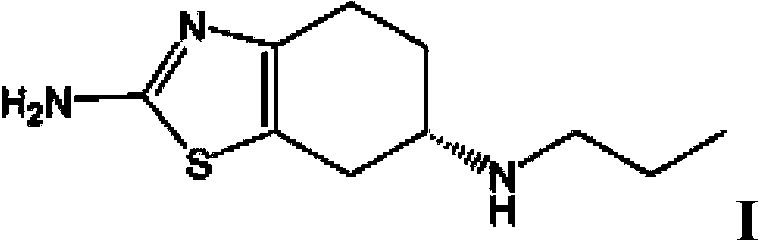
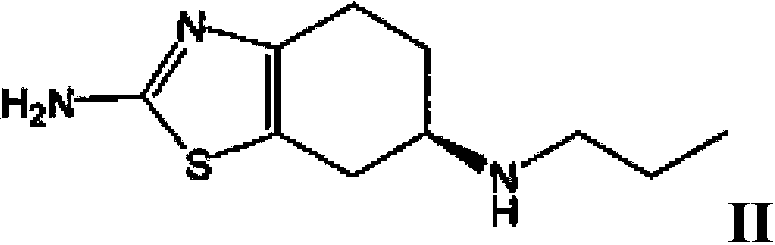
ActiveCN103435571AFully intrinsically activeRapid and complete oral absorptionOrganic active ingredientsNervous disorderSolventNervous system disease
The invention discloses a tetrahydrobenzothiazole derivate for treating nerve diseases. Particularly, the tetrahydrobenzothiazole derivate comprises compounds shown in the following formula I: (S)-(-)-2-amino-6-(propionamide)-4,5,6,7- tetrahydrobenzothiazole, acceptable salt of the compounds in pharmacy or solvates of the compounds. The compounds or compositions of the tetrahydrobenzothiazole derivate can be used for treating nervous system diseases.
Owner:SHANDONG LUKANG PHARMACEUTICAL GROUP SAITE CO LTD
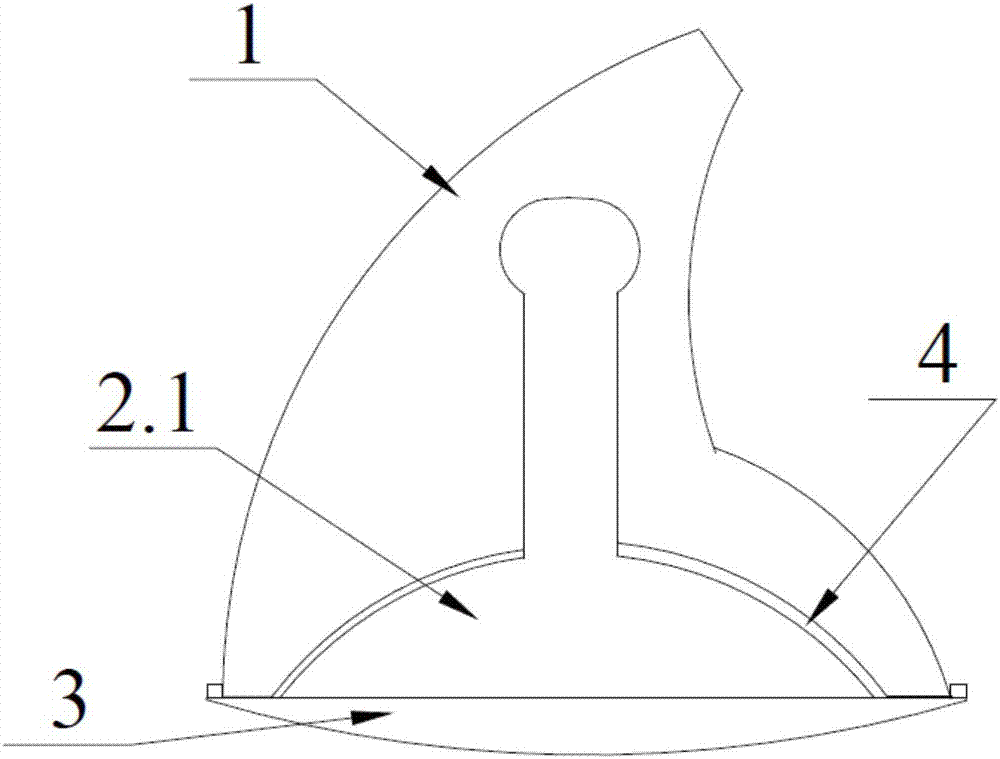
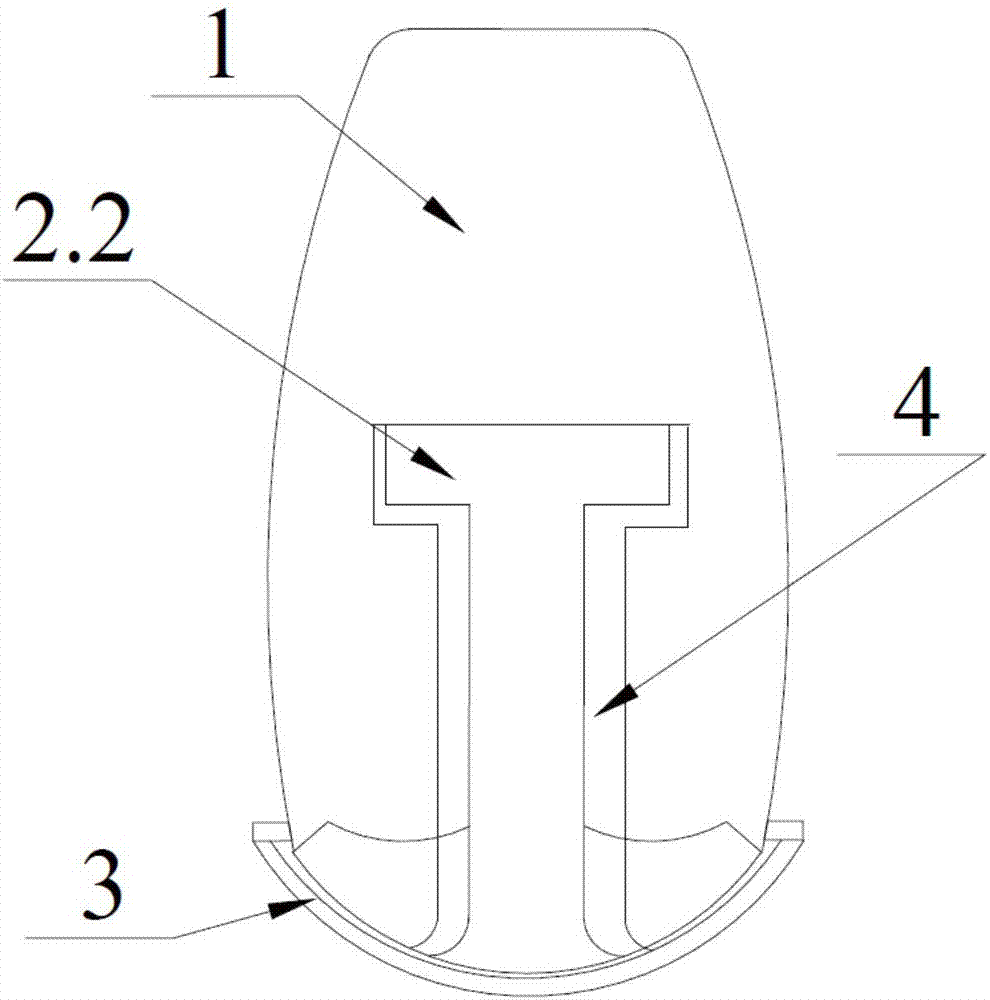
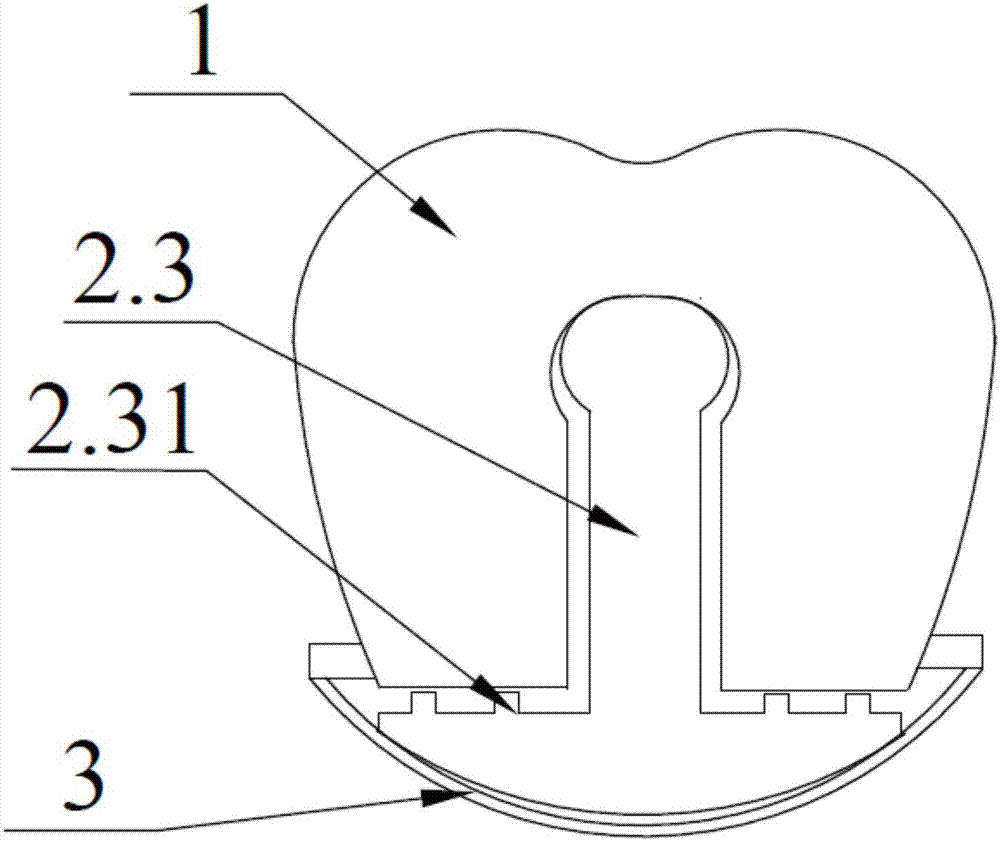
Complete denture movable flap and assembling method thereof
InactiveCN107137154AAvoid comfortRecovery fretting rangeArtificial teethDental surgeryDenturesEngineering
The invention discloses a complete denture movable flap and an assembling method thereof. The complete denture movable flap comprises a cap crown portion, a buffer supporting portion and a base bracket portion. The buffer supporting portion is of a columnar structure, the bottom of which is connected to the base bracket and the upper portion of which is provided with a locking positioning end; the external structure of the cap crown portion is similar to the shape of a tooth; transverse through grooves are formed in the lower portion and the central position of the cap crown portion, and the shape of the groove is matched with that of a side face of the buffer supporting portion. The assembling method comprises the following steps: firstly, processing the cap crown portion, the buffer supporting portion and the base bracket portion through a mould; then sleeving the buffer supporting portion with the cap crown portion from the side face through a through groove; then locking or clamping the bottom of the buffer supporting portion on the base bracket; and finally, clamping or embedding the base bracket portion to a denture. The complete denture movable flap disclosed by the invention solves the problem of comfort of a patient with false teeth in chewing food and the problem of alleviating guided excessive absorption of residual alveolar bones by yawing forces of the false teeth and etc.
Owner:王朝阳
Cross-linked environment-friendly flame-retardant reinforced polyamide-based composite material and preparation method thereof
InactiveCN111334036AGood physical and mechanical propertiesImprove mechanical propertiesGlass fiberHeat deflection temperature
The invention discloses a cross-linked environment-friendly flame-retardant reinforced polyamide-based composite material and a preparation method thereof. The material comprises, by weight, 20 to 80parts of aliphatic polyamide, 10-40 parts of glass fibers, 10 to 20 parts of a flame retardant, 0.5 to 10 parts of a main cross-linking agent, 0.5 to 10 parts of an auxiliary cross-linking agent, 0.5to 10 parts of a compatilizer, 0.1 to 0.3 part of a coupling agent, 0.1 to 0.3 part of a lubricating agent and 0.1 to 0.3 part of an antioxidant. The mechanical properties and thermal properties, especially the thermal deformation temperature, of the cross-linked environment-friendly flame-retardant reinforced polyamide-based composite material prepared from the raw materials are integrally improved by 30% or above; the mechanical property and the thermal property, especially the thermal deformation temperature, are integrally improved by 15% or above, the strength of the weld line is improvedby adopting the preparation method, and the risk of damage caused by cracks in the use process of the material is reduced to a great extent.
Owner:河北旭阳能源有限公司 +2
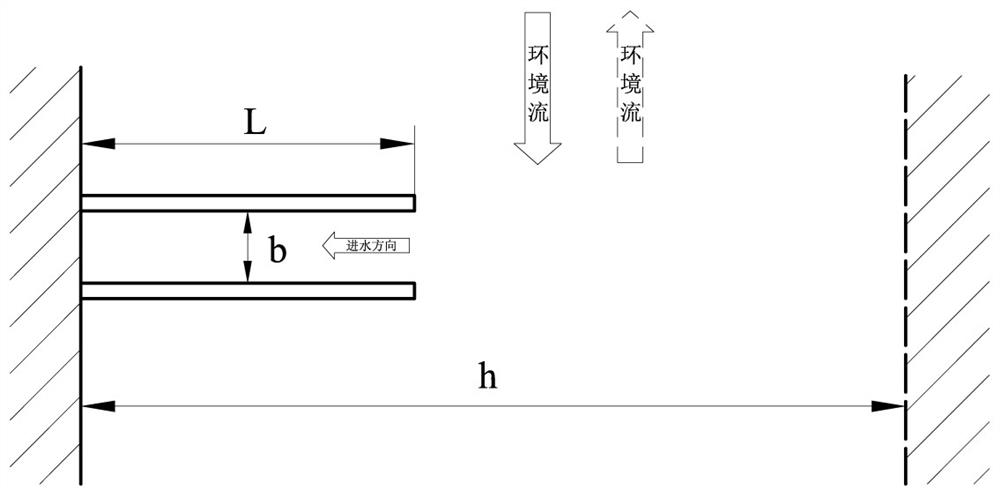
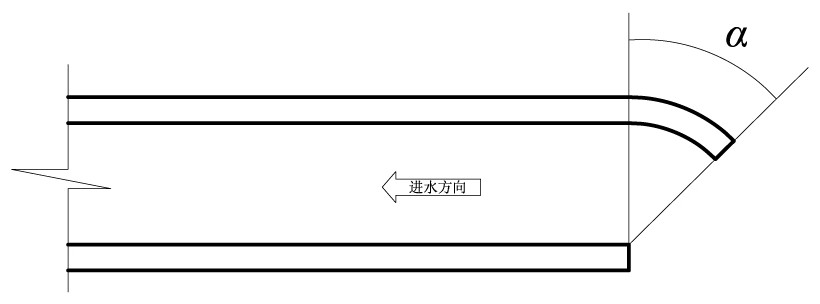
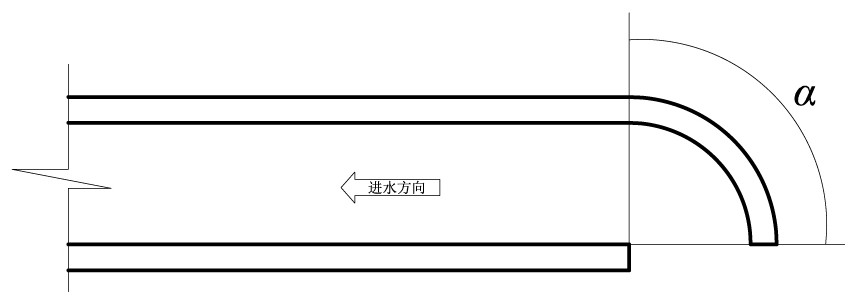
Power plant water intake open channel layout analysis method based on reduction of water intake entrainment effect
ActiveCN113901557AReduce the impact of water intake entrainmentReduce entrainmentGeometric CADConstraint-based CADNuclear plantMathematical model
The invention relates to a power plant water intake open channel layout analysis method based on reduction of a water intake entrainment effect. The method comprises the following steps: constructing a three-dimensional mathematical model of a water intake open channel of a nuclear power plant; analyzing internal and external flow fields of the water taking open channel; constructing a water taking open channel organism and sundry throwing simulation mathematical model; analyzing the water taking entrainment rate of organisms and sundries in the water taking open channel; and comprehensively analyzing the water taking entrainment rates of the water taking open channels with different structures. Aiming at a water taking open channel structure of a nuclear power plant, according to environmental characteristics such as water area flow velocity, flow direction, terrain and shoreline of a water taking project, flow field characteristics inside and outside the water taking open channel structure are fully utilized, the entrainment effect of water taking on organisms and sundries is analyzed, and the water taking safety is improved. According to the method, the internal and external flow field distribution characteristics and the water taking entrainment rate of the water taking open channel in the water taking engineering water area are provided, and a basis is provided for reducing the water taking entrainment influence of the open channel.
Owner:CHINA INST OF WATER RESOURCES & HYDROPOWER RES
Mirabegron slow-release mini-pill and preparation method thereof
InactiveCN105687163AThe treatment effect is stableLarge distribution areaOrganic active ingredientsPharmaceutical non-active ingredientsMirabegronUrge urinary incontinence
A mirabegron slow-release mini-pill includes a drug-containing mini-pill and a coating layer, the drug-containing mini-pill is coated with the coating layer, the drug-containing mini-pill comprises 25mg of mirabegron, 70mg of a blank pill core, 100-200mg of a filler, 50-150mg of a lubricant and 5-50mg of a binder, the coating layer comprises 35-175mg of Eudragit NE30D and 5-52mg of talc powder, preparation steps are as follows: 1, preparing materials; 2, mixing; 3, preparing the binder; 4, pelleting; 5, preparing a coating agent; 6, coating; 7, filling; and 8, aluminum molding and finishing. The mirabegron slow-release mini-pill is used for urge urinary incontinence, dibetes insipidus overactive bladder, two advanced technologies of a novel slow-release preparation and a mini-pill preparation are used, treatment effect is stable, bioavailability is higher, the mirabegron slow-release mini-pill has the advantages of good drug stability, convenient packaging, transportation, storage and the like, and the preparation method is simple and suitable for industrial production.
Owner:HEILONGJIANG ZHICHENG MEDICAL TECH
Choline fenofibric acid sustained release pellets and preparation method thereof
InactiveCN104434847AIncreased surface distribution areaReduce absorption rateOrganic active ingredientsMetabolism disorderSustained release pelletsFENOFIBRIC ACID
The invention provides choline fenofibric acid sustained release pellets. The choline fenofibric acid sustained release pellets comprise medicine-containing pellets and coating layers, wherein the medicine-containing pellets are coated with the coating layers; the medicine-containing pellets comprise 45mg of choline fenofibric acid, 100-200mg of hollow cores, 100-200mg of filling agents, 25-125mg of lubricating agents and 5-50mg of bonding agents; the coating layers comprise 35-175mg of Eudragit NE30D and 5-52mg of talcum powder. A preparation method of the choline fenofibric acid sustained release pellets comprises the following processes: 1. material preparation; 2. mixing; 3. preparation of the bonding agents; 4. preparation of the pellets; 5. preparation of coating agents; 6. coating; 7. filling; 8. aluminium-plastic packaging and preparation of finished products. The choline fenofibric acid sustained release pellets used for reducing blood lipid have the beneficial effects that as the two kinds of advanced technologies, namely novel sustained release preparations and pellet preparations, are adopted, the choline fenofibric acid sustained release pellets have stable treatment effects and higher bioavailability and have the advantages of good medicine stability, convenience in packaging, transportation and storage, and the like. The preparation method of the choline fenofibric acid sustained release pellets is simple and practicable and is suitable for industrial production.
Owner:HARBIN SHENGJI PHARMA
Environment-friendly food freezing device
PendingCN110822784AReduce heat exchangeReduce stepsMechanical apparatusDomestic refrigeratorsCold airThermodynamics
The invention discloses an environment-friendly food freezing device, and belongs to the field of food freezing. The device comprises a freezer, a first shell is fixed in the freezer, a mounting frameis fixed in the first shell, an evaporator is mounted on the mounting frame, a second shell is fixed on the side wall of one side of the freezer, a compressor is installed at the inner bottom of thesecond shell, a condenser and a capillary are fixed in the second shell, an air outlet of the compressor is connected with the condenser, the air inlet of the compressor is connected with a first conduit, the other end of the first conduit is connected with the evaporator, the air outlet of the condenser is connected with the capillary, and the air outlet of the capillary is connected with the evaporator. According to the environment-friendly food freezing device, heat exchange can be effectively reduced, the freezing liquid is subjected to secondary cooling through the evaporator providing cold air, energy is saved, cost is saved, the freezing process is continuous and efficient, residual freezing liquid collection circulation can be carried out in time, operation steps are reduced, and work efficiency is improved.
Owner:连云港市桂柳食品有限公司
Preparation method and application of biological medicinal bait for trionyx sinensis
InactiveCN106390134AAbsorption rate changeReduce absorption rateTetracycline active ingredientsClimate change adaptationSolventDrug
The invention discloses a preparation method and application of biological medicinal bait for trionyx sinensis. The preparation method includes the steps of A, selecting a biological carrier A; B, weighting medicine B, using a formula to calculate the dosage of the weighed medicine, and using a solvent C to dissolve the medicine; C, adding phagostimulant D into the medicine solution, and using distilled water to dilute the mixed liquid; D, using a glass syringe to extract the medicine solution; E, injecting the medicine solution into the biological carrier A to obtain the biological medicinal bait. The invention further discloses application of the biological medicinal bait to the pharmacological study of the trionyx sinensis. The biological medicinal bait has the advantages that the biological medicinal bait is simple in preparation process and convenient to operate, the phagostimulant is added into the medicine solution to induce the trionyx sinensis to eat the biological medicinal bait, the special smell of the medicine can be masked, and the trionyx sinensis can easily accept the biological medicinal bait; the actual absorption state of the medicine in the intestinal track of the trionyx sinensis can be disclosed favorably; the biological medicinal bait is suitable for applying the medicine to the trionyx sinensis through the biological carrier, and the pharmacological effect of the medicine in the trionyx sinensis can be researched.
Owner:YANGTZE RIVER FISHERIES RES INST CHINESE ACAD OF FISHERY SCI
Magnesium valproate sustained release tablet and preparation process thereof
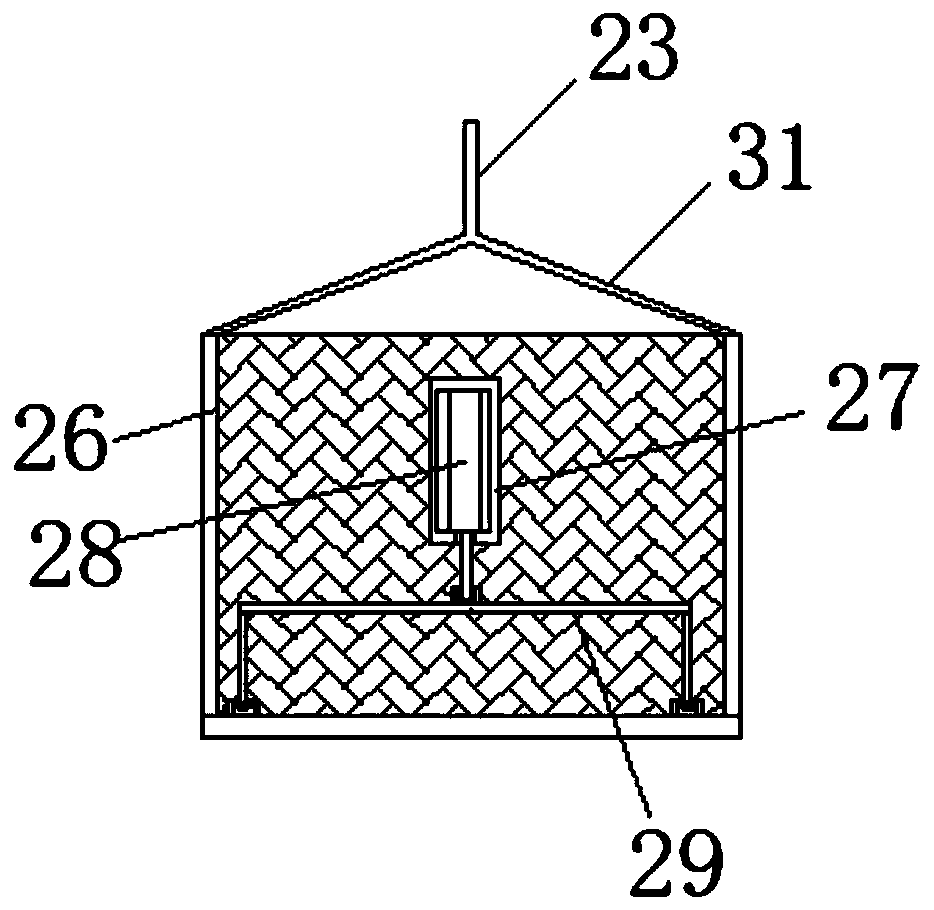
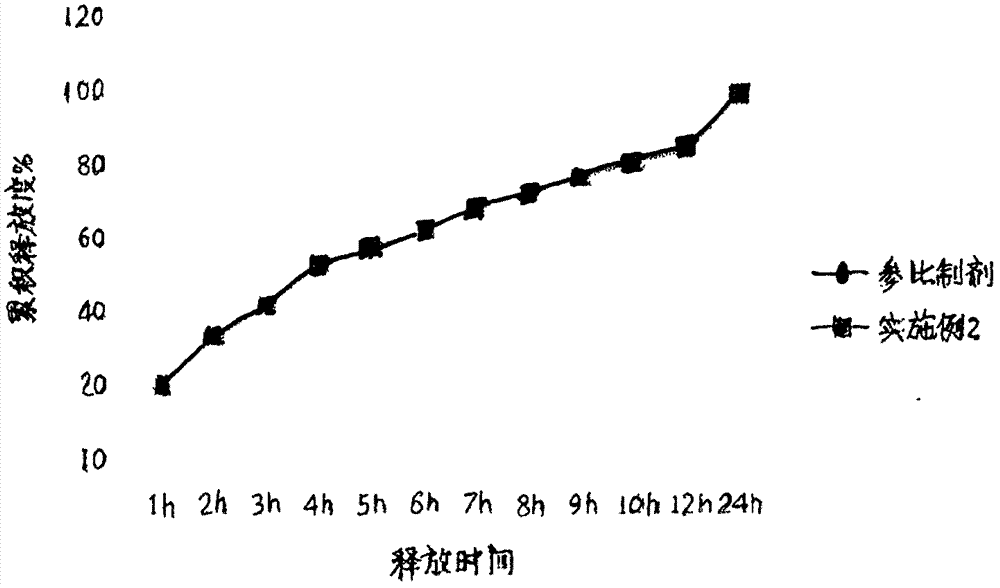
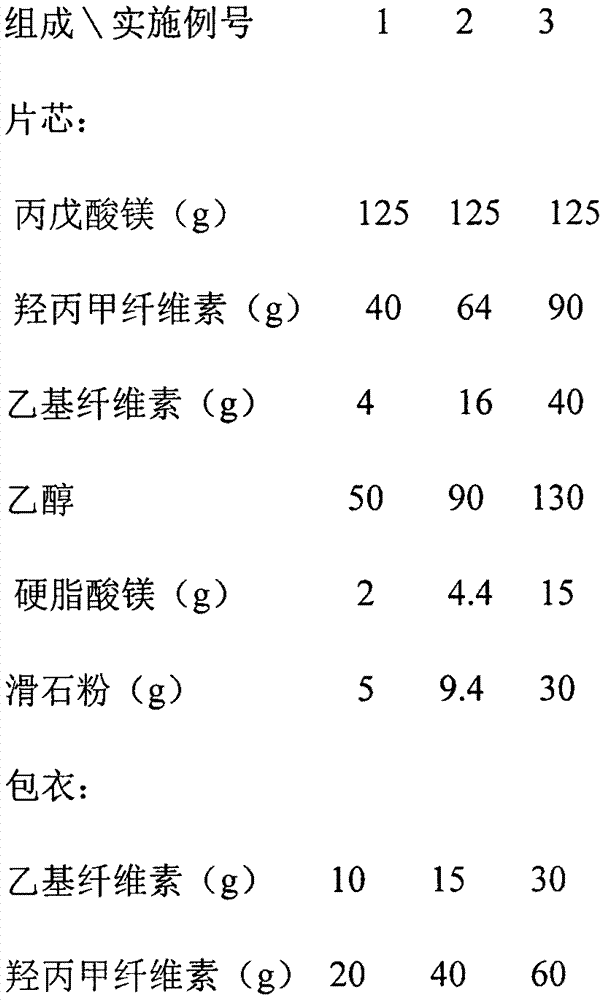
InactiveCN107875133AImprove complianceReduce the number of dosesNervous disorderPharmaceutical non-active ingredientsSustained Release TabletAlcohol
The invention relates to a magnesium valproate sustained release tablet and a preparation process thereof. The magnesium valproate sustained release tablet is prepared from the following two parts: A,a tablet core prepared from the following substances: 29 to 54 percent of magnesium valproate, 17 to 21 percent of hydroxypropyl methylcellulose, 2 to 9 percent of ethylcellulose, 22 to 30 percent ofethyl alcohol, 0.8 to 4 percent of magnesium stearate, and 3 to 7 percent of talcum powder; B, a sustained release tablet coating formula prepared from the following substances: 0.7 to 2 percent of ethylcellulose, 0.7 to 1.3 percent of glycerinum, 1.3 to 4 percent of hydroxypropyl methylcellulose, and 0.3 to 1.3 percent of talcum powder. The preparation process comprises: A, a preparation processof the tablet core, comprising the steps of weighing raw materials according to tablet core formula amounts uniformly mixing, adding an appropriate amount of the ethyl alcohol, preparing a soft material, sieving and pelletizing, then baking and drying at the temperature of 55 DEG C to 65 DEG C, adding the magnesium stearate and the talcum powder, uniformly mixing, measuring the content, and pressing to form an oval tablet; B, a preparation process of the coating comprising the steps of weighing ingredients according to sustained release tablet coating formula amounts, dissolving through an appropriate amount of 50 to 100 percent ethyl alcohol, and then spraying a coating on the tablet core.
Owner:湖南省湘中制药有限公司
Nanoparticulate compositions of tubulin inhibitor compounds
InactiveCN101090720AIncrease doseLarge doseOrganic active ingredientsPharmaceutical delivery mechanismTubulin InhibitorsWater soluble
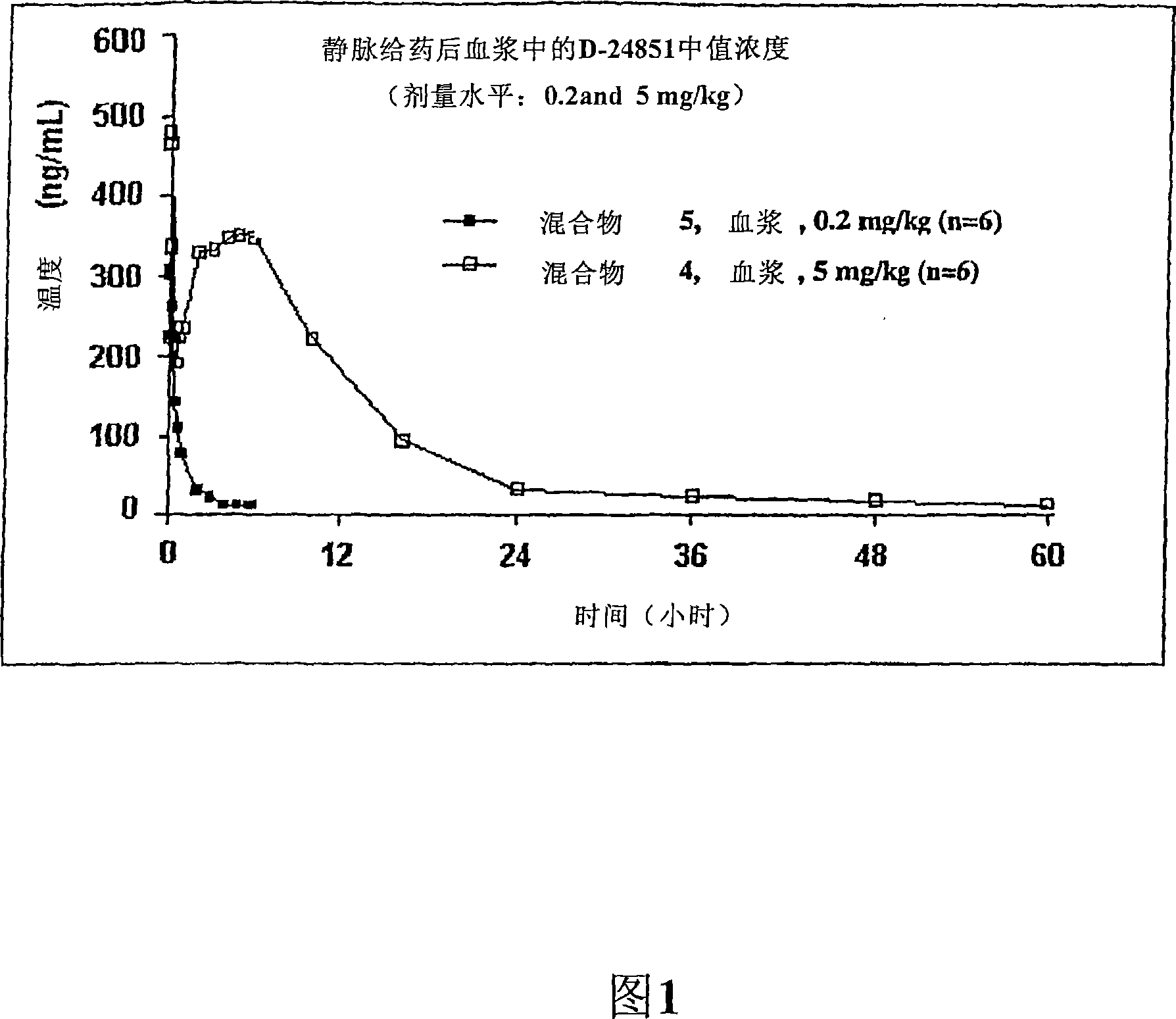
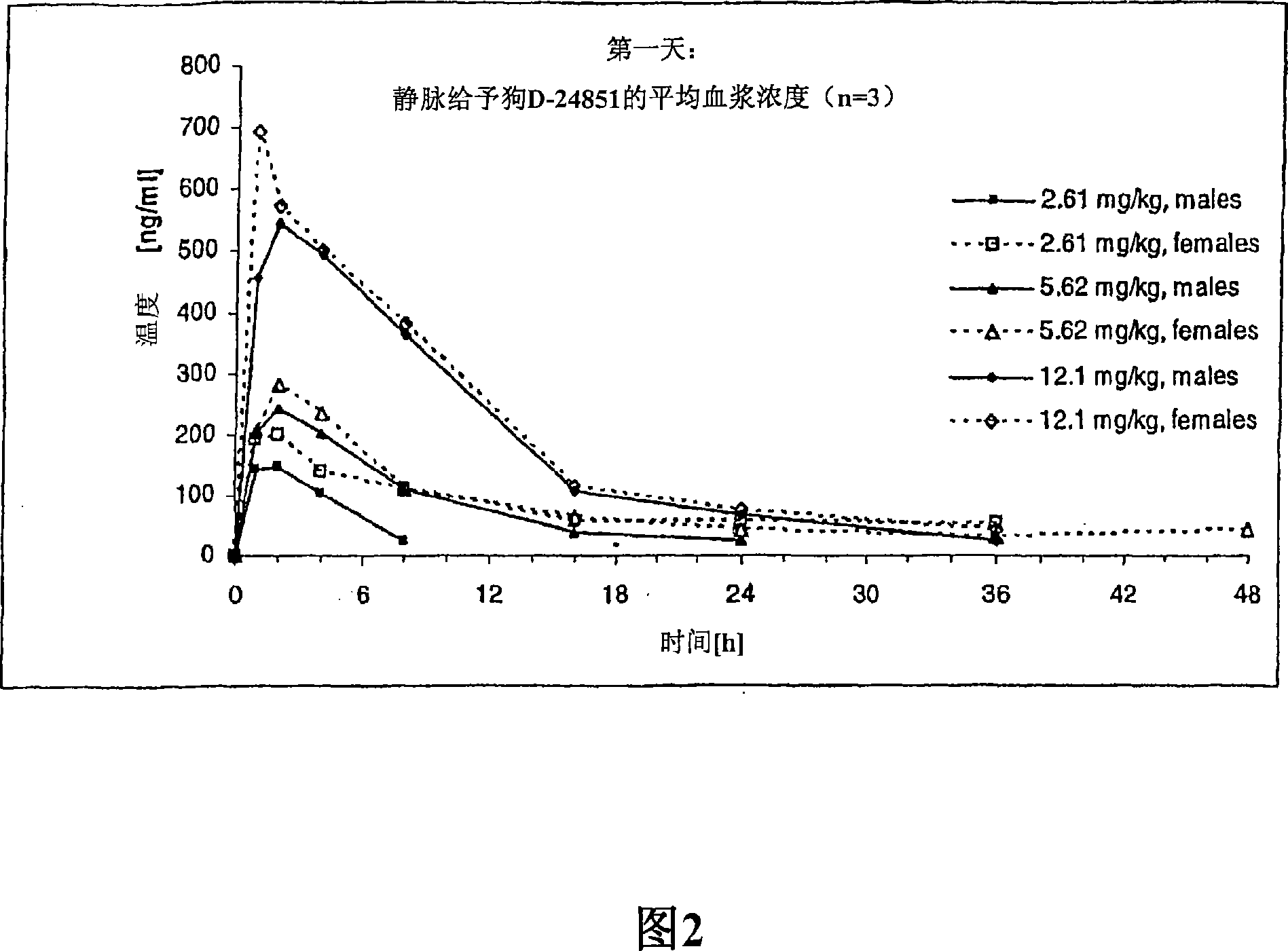
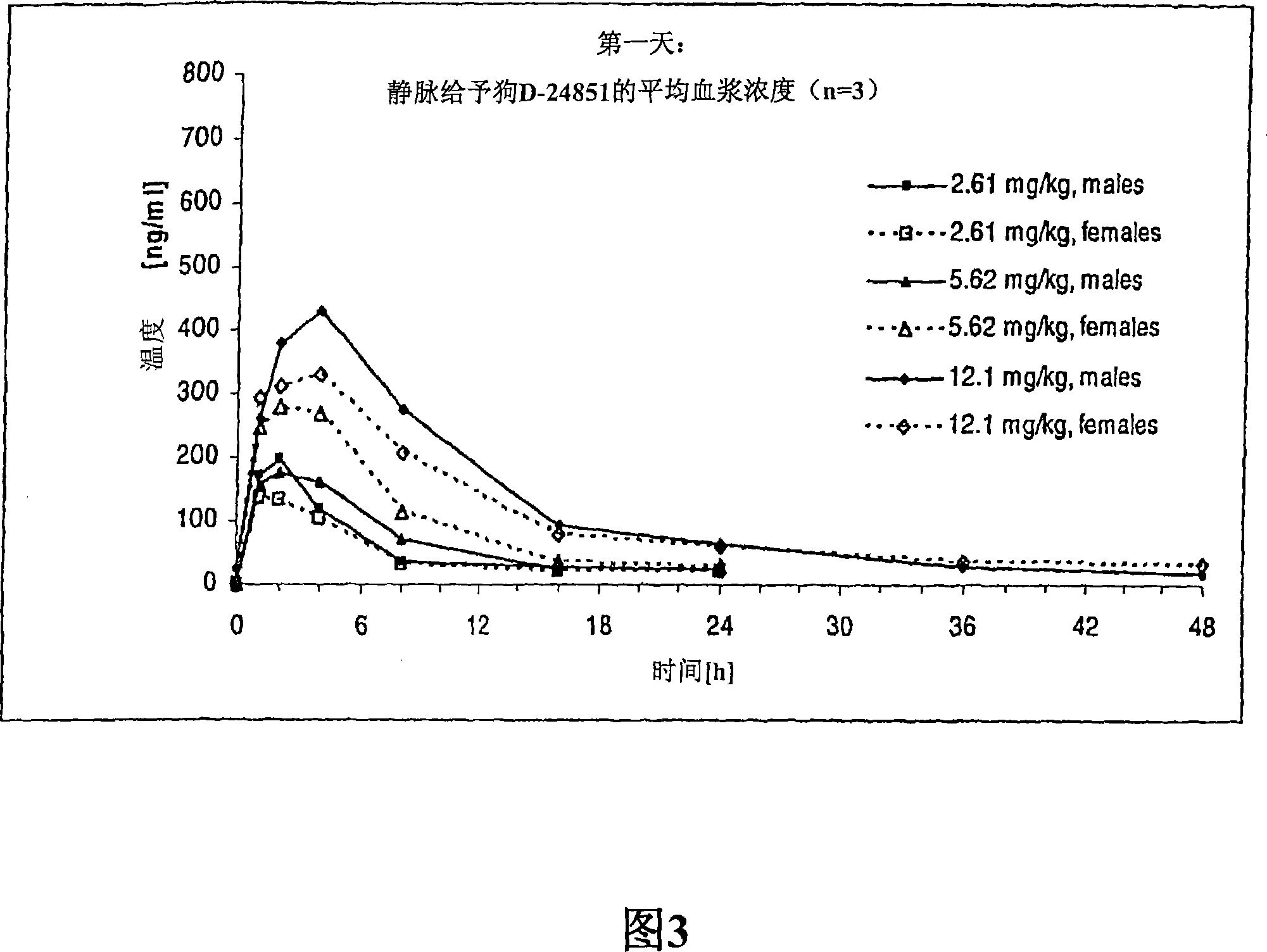
The present invention is directed to novel pharmaceutical compositions comprinsing nano- and micro-particulate formulations of poorly water soluble tubulin inhibitors of the indole chemical class, preferably N-substituted indol-3-glyoxyamides, and more preferably N-(Pyridin-4-yl)-[1-(4-chlorobenzyl)-indol-3-yl]glyoxylic acid amide (D-24851), also known as ''Indibulin'', and methods of making and using such compositions for the treatment of anti-tumor agent resistant cancers and other diseases.
Owner:BAXTER INT INC +1
Apixaban sustained-release tablet and preparation method thereof
InactiveCN105596309AReduce absorption rateAbsorb evenlyOrganic active ingredientsPharmaceutical delivery mechanismDrug release rateProlonged-release tablet
The invention discloses an apixaban sustained-release tablet and a preparation method thereof. The apixaban sustained-release tablet comprises the following components: apixaban, hydroxypropyl methylcellulose, and a lubricant. The apixaban sustained-release tablet employs a novel sustained-release preparation and a pellets preparation, sustained release means that by prolonging drug release rate of a drug in a dosage form, the absorption rate of the drug in human body is reduced, so that more stable treatment effect can be realized; the tablet has the advantage of large distribution area of the drug on surface of gastrointestinal tract, reduces irritation, increases bioavailability, and does not be influenced by gastric emptying influence, the drug can be uniformly absorbed by human body, individual difference is little; simultaneous application of two advanced technologies enhances the technical advantage of the apixaban sustained-release tablet; compared with oral liquid, the apixaban sustained-release tablet has the advantages of good drug stability, convenient package, transport and storage, the preparation method is simple and easy to operate, and is suitable for industrial production.
Owner:HARBIN SHENGJI PHARMA
ptsG gene knocked out recombination bacterial efficiently expressing human-like collagen protein, construction method thereof, and protein expression
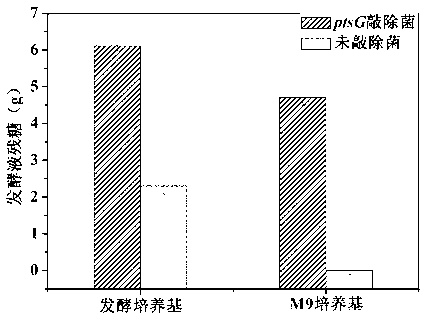
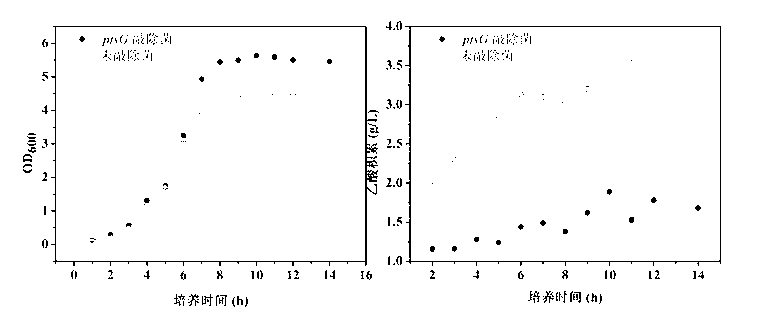
ActiveCN103224901AReduce absorption rateBalanced utilizationBacteriaMicroorganism based processesEscherichia coliGenetic engineering
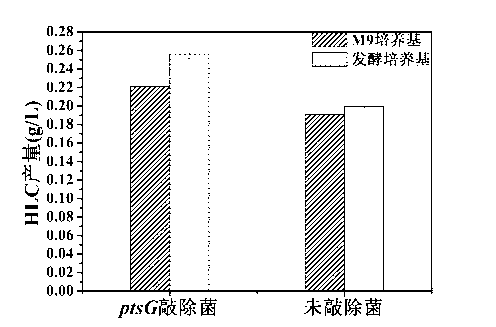
The invention relates to ptsG gene knocked out recombination bacterial efficiently expressing human-like collagen protein, a construction method thereof, and protein expression. In the prior art, when escherichia coli is adopted to carry out fermentation production of human-like collagen protein, acetic acid accumulation can affect bacterial growth and protein expression, and a collagen protein yield can be reduced with the existing acetic acid byproduct reduction method. According to the present invention, escherichia coli BL21 with a preservation number of CGMCC No.0743 is adopted as starting bacterial, Red homologous recombination is adopted, and apramycin resistance gene is adopted to replace ptsG gene on the escherichia coli genome, and FLP incision enzyme expressed by plasmid pCP20 is adopted to eliminate the apramycin resistance gene to obtain the ptsG gene knocked out escherichia coli engineering bacterial CGMCC No.7331. According to the present invention, a gene engineering tool is adopted to transform an escherichia coli glucose absorption way so as to reduce acetic acid accumulation, improve human-like collagen accumulation, reduce glucose consumption by 9-28%, and improve human-like collagen protein yield by 20-30%.
Owner:NORTHWEST UNIV(CN)
Magnesium valproate sustained-release tablet and preparing method thereof
InactiveCN105456218AReduce absorption rateSmall toxicityNervous disorderPharmaceutical non-active ingredientsSide effectMagnesium Valproate
The invention discloses a magnesium valproate sustained-release tablet and a preparing method thereof. The magnesium valproate sustained-release tablet is prepared from magnesium valproate, hydroxypropyl methylcellulose and a lubricating agent through the process steps of material preparing, mixing, granulating, total mixing, tabletting, aluminum-plastic packaging and the like. The magnesium valproate sustained-release tablet mainly has the antiepileptic and anti-mania treatment effects. A novel sustained release preparation is adopted, according to the definition of sustained release, the absorption efficiency of a drug entering an organism is reduced by reducing the drug release speed of the drug in the dosage form, and therefore a more stable treatment effect is achieved; the effective blood concentration can be kept in a long time, the toxic and side effects of the drug can be reduced, and drug use safety is improved. The drug is convenient to use and particularly suitable for patients with chronic diseases and improves compliance of patients. The magnesium valproate sustained-release tablet has the advantages of being high in stability, convenient to package, transport and store and the like, and the preparing method is simple, easy to implement and suitable for industrial production.
Owner:HEILONGJIANG ZHICHENG MEDICAL TECH
High efficiency air filter
InactiveCN102078733AIncrease storage spaceSmall amount of deformationSuction filtersDispersed particle filtrationAir filterEngineering
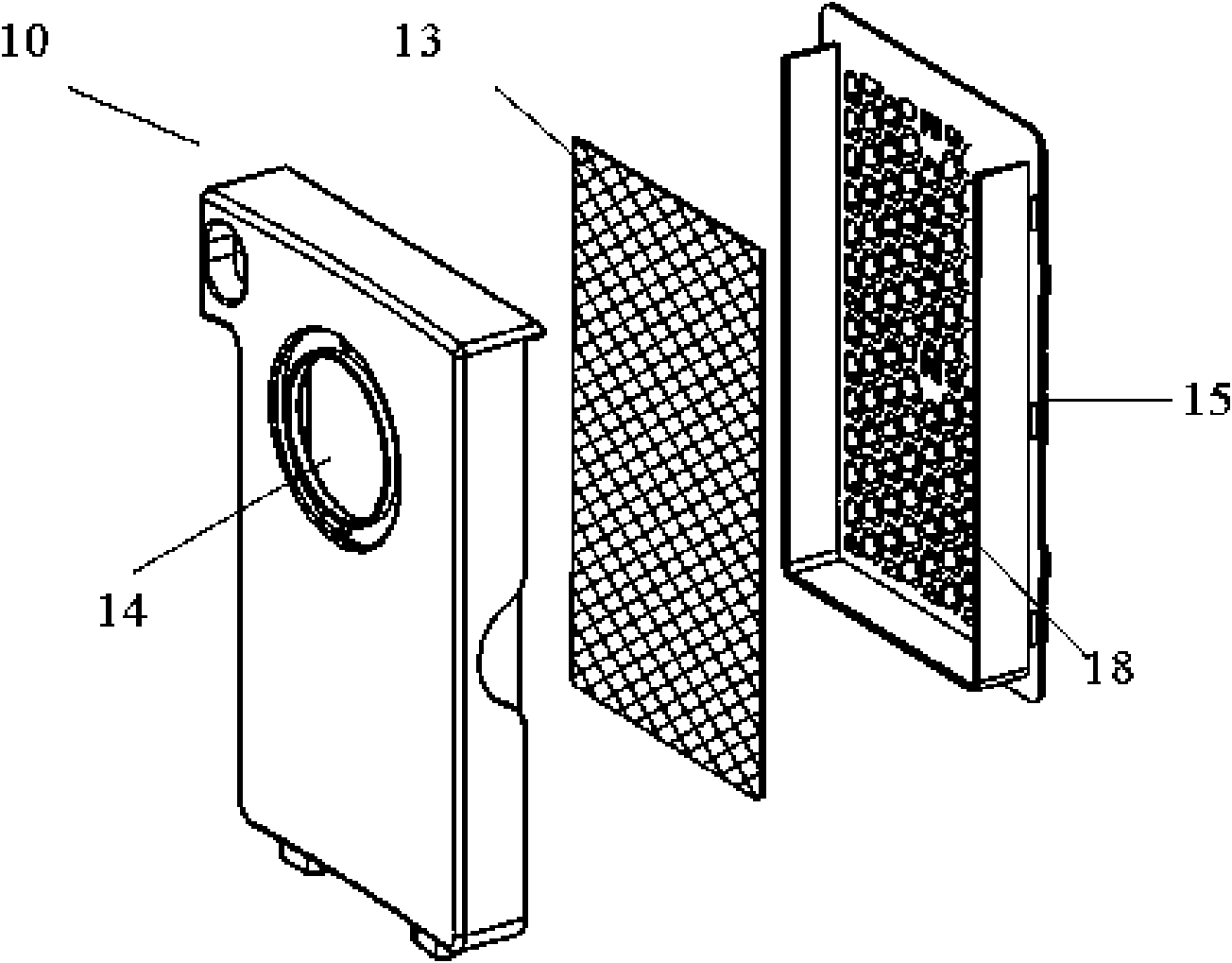
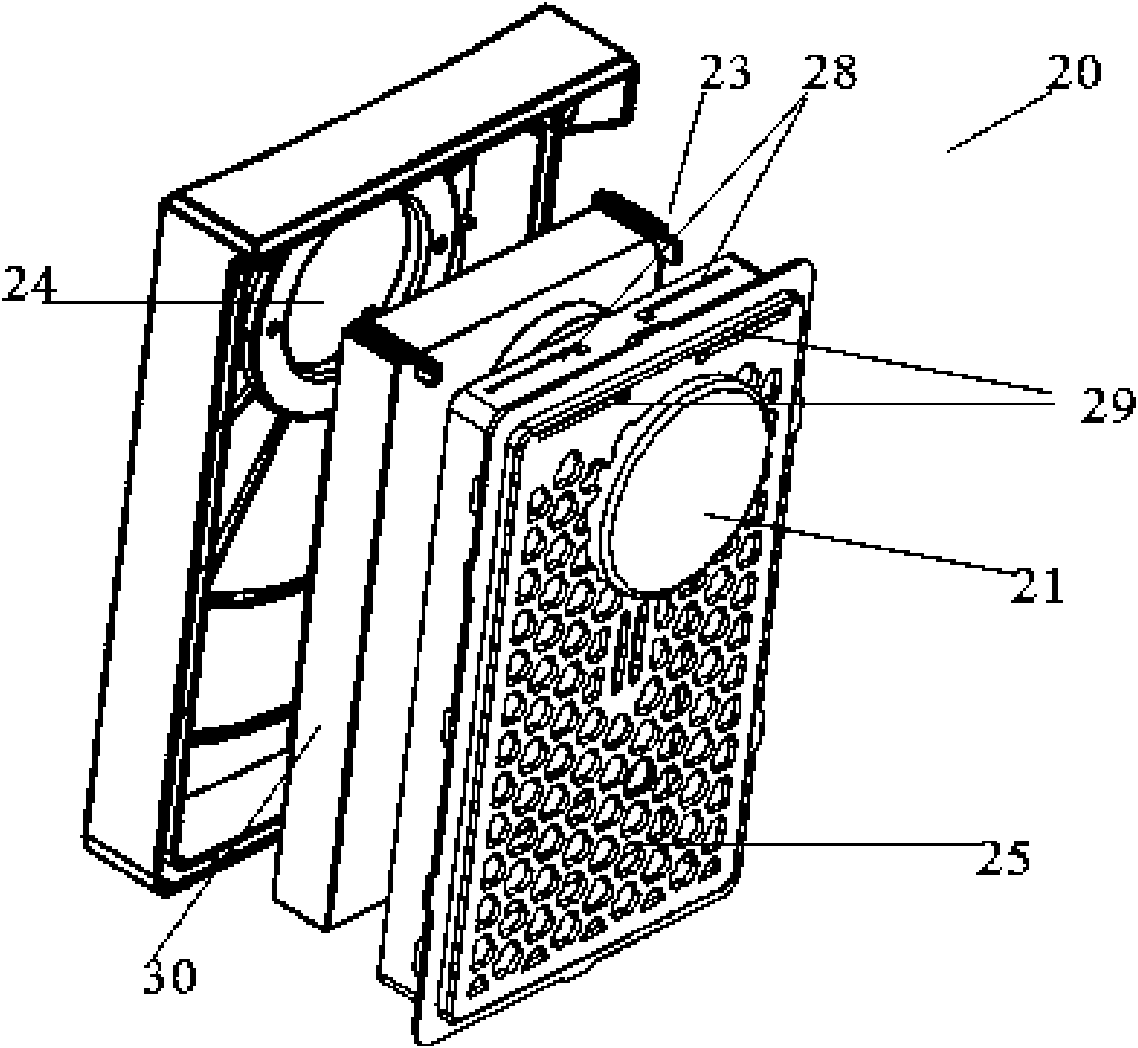
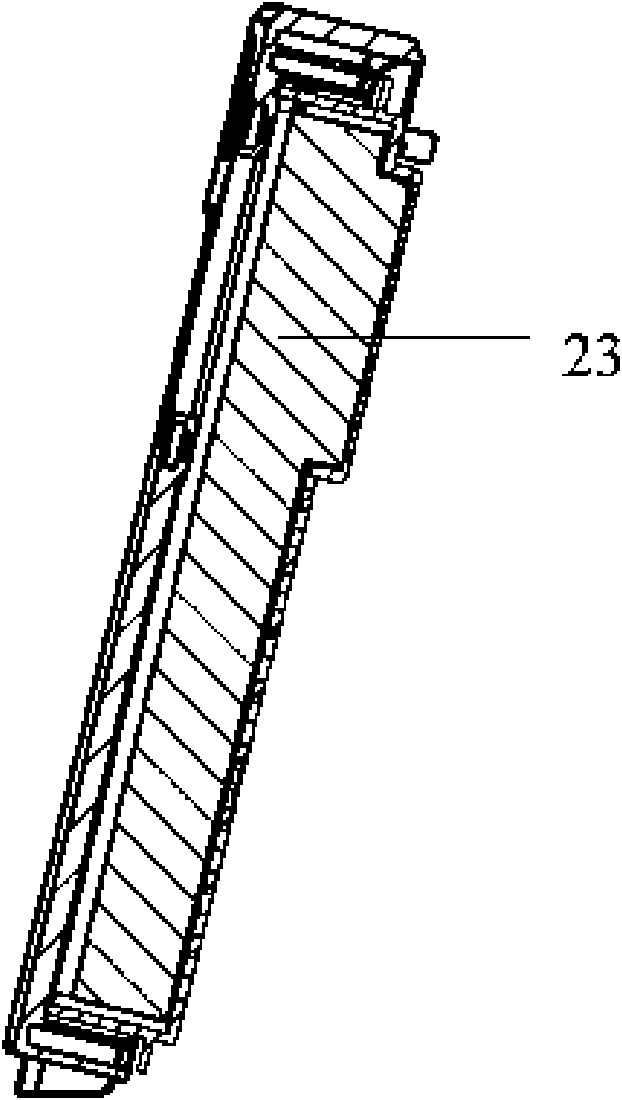
The invention discloses a high efficiency air filter which is in a box-shaped structure and is characterized in that an air inlet is formed on a front wall plate, a rear cover grid is provided with an exhaust hole and is internally provided with a filtering material, a buffer cavity is formed in an externally convex manner on the grid corresponding to the air inlet, and two sides of the top of the grid are symmetrically provided with transverse guide slots; the filtering material is fixedly connected with a sheet which is fixedly connected with one end of a brake arm, a free end of the brake arm stretches to the outer side of the rear cover grid through the guide slots, and the brake arm can slide inside the guide slots. The grid is provided with the convex buffer cavity which can shunt the incoming flow, and simultaneously, a structure for manually shaking the filtering material is arranged, so the residual dust in sponge can be shaken off by manual compression and vibration, a matching structure for a guide plate and a positioning guide slot can connect a rear cover with a front cover so as to reduce the deformation of the front cover and the rear cover during engagement thereof, and with the help of the manual structure, a cover of the filter can be easily opened without dust falling; and the high efficiency air filter is simple in structure and convenient in operation, and enhances user satisfaction.
Owner:LG ELECTRONICS (TIANJIN) APPLIANCES CO LTD
Alfuzosin hydrochloride sustained release tablets and preparation method thereof
InactiveCN105287422AReduce absorption rateSmall toxicityOrganic active ingredientsPharmaceutical delivery mechanismAlfuzosinBlood drug concentration
The invention discloses alfuzosin hydrochloride sustained release tablets and a preparation method thereof. The sustained release tablets are prepared by adopting the following raw materials: alfuzosin hydrochloride, hydroxypropyl methylcellulose and lubricating agents, and adopting the processing steps of material preparation, mixing, granulation, total blending, tabletting, aluminium-plastic packaging, and the like. The alfuzosin hydrochloride sustained release tablets have the beneficial effects that the alfuzosin hydrochloride sustained release tablets are mainly used for treating functional symptoms of benign prostatic hyperplasia; the more novel sustained release preparation is adopted; sustained release refers to that the rate of absorption of the medicines into bodies is reduced by reducing the rate of release of the medicines from the dosage form, thus achieving the more stable treatment effects; the effective blood concentration can be maintained in a longer time, the toxic and side effects of the medicines can be also reduced and the medicine safety is improved; the alfuzosin hydrochloride sustained release tablets are convenient to use, are especially suitable for chronic disease patients who take medicines for a long term, and have the effect of improving the compliance of the patients; alfuzosin hydrochloride sustained release tablets have the advantages of good medicine stability, convenience in packaging, transportation and storage, and the like; and the preparation method is simple and practicable and is suitable for industrial production.
Owner:HEILONGJIANG ZHICHENG MEDICAL TECH
Application of apixaban to medicine for treating diabetic foot
InactiveCN106902115AEasy to takePromote absorptionOrganic active ingredientsMetabolism disorderThrombusDiabetic foot
The invention relates to novel application of a medicine, and in particular relates to a treatment effect of apixaban on diabetic foot. A clinical trial research provided by the invention proves that the apixaban used as a reversible, direct and high-selectivity Xa factor active site inhibitor which is potent and is orally available can be used for inhibiting free and thrombus-combined Xa factors and inhibiting prothrombinase activity. By inhibiting the Xa factors, the apixaban can be used for inhibiting the generation of thrombin and inhibiting the formation of thrombus; erythrocyte deformability is further improved, the blood and plasma viscosity is reduced, and acra blood circulation is promoted, so that the apixaban has a good treatment effect on the diabetic foot.
Owner:JIANGSU WANBANG BIOPHARMLS +1
Doxofylline sustained-release pellets and preparation method thereof
InactiveCN105640921AReduce absorption rateStable absorptionPharmaceutical delivery mechanismPharmaceutical non-active ingredientsDoxofyllineAdhesive
The invention provides doxofylline sustained-release pellets. The doxofylline sustained-release pellets comprise medicine-containing pellets and coating layers, wherein the medicine-containing pellets are coated by the coating layers; the medicine-containing pellets comprise 200 mg of doxofylline, 70 mg of hollow pellet cores, 110-160 mg of a filling agent, 18-68 mg of a lubricating agent and 2-10 mg of an adhesive; the coating layers comprise 45-225 mg of Eudragit NE30D and 7-68 mg of talcum powder. A preparation method of the doxofylline sustained-release pellets comprises the following processes: 1, material preparation; 2, mixing; 3, preparation of the adhesive; 4, preparation of the pellets; 5, preparation of a coating agent; 6, coating; 7, filling; and 8, aluminum-plastic packaging and preparation of finished products. The doxofylline sustained-release pellets which can effectively treat bronchial asthma, asthmatic chronic bronchitis and dyspnea and other symptoms caused by bronchospasm is provided; as two kinds of advanced technologies, namely novel sustained-release preparations and pellet preparations, are adopted, the doxofylline sustained-release pellets have stable treatment effects and higher bioavailability and have the advantages of good medicine stability, convenience in packaging, transportation and storage and the like; the preparation method is simple and practicable and is suitable for industrial production.
Owner:HARBIN SHENGJI PHARMA
Tamsulosin hydrochloride sustained-release pellet and preparation method thereof
InactiveCN105412020AReduce absorption rateAbsorb evenlyUrinary disorderAmide active ingredientsSustained release pelletsIrritation
The invention relates to a tamsulosin hydrochloride sustained-release pellet. The tamsulosin hydrochloride sustained-release pellet comprises a medicine-containing pellet body and a coating layer, wherein the coating layer wraps the medicine-containing pellet body, the medicine-containing pellet body comprises tamsulosin hydrochloride, a blank pellet core, a filling agent, a lubricating agent and an adhering agent, and the coating layer comprises Eudragit NE30D and talcum powder. A preparation method comprises the following steps: 1, preparing materials; 2, mixing; 3, preparing the adhering agent; 4, pelleting; 5 preparing a coating agent; 6, coating; 7, filling; and 8, packaging by aluminum-plastic for obtaining a finished product. The tamsulosin hydrochloride sustained-release pellet is suitable for the symptoms such as urination disorder caused by benign prostatic hyperplasia, and is good in drug release stability, small in irritation to the gastrointestinal tract, good in bioavailability, convenient to package, transport and store, and suitable for industrial production. The tamsulosin hydrochloride sustained-release pellet is good in absorption after oral medication, and although the tamsulosin hydrochloride sustained-release pellet can be taken on an empty stomach or after the meal, food can increase the total absorptive amount of the tamsulosin hydrochloride sustained-release pellet.
Owner:HEILONGJIANG ZHICHENG MEDICAL TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com