Magnesium valproate sustained release tablet and preparation process thereof
A magnesium valproate, preparation technology, applied in anhydride/acid/halide active ingredients, coatings, pill delivery, etc., can solve the problems of patients who are difficult to persist for a long time, high peak blood concentration, and large fluctuations in blood concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
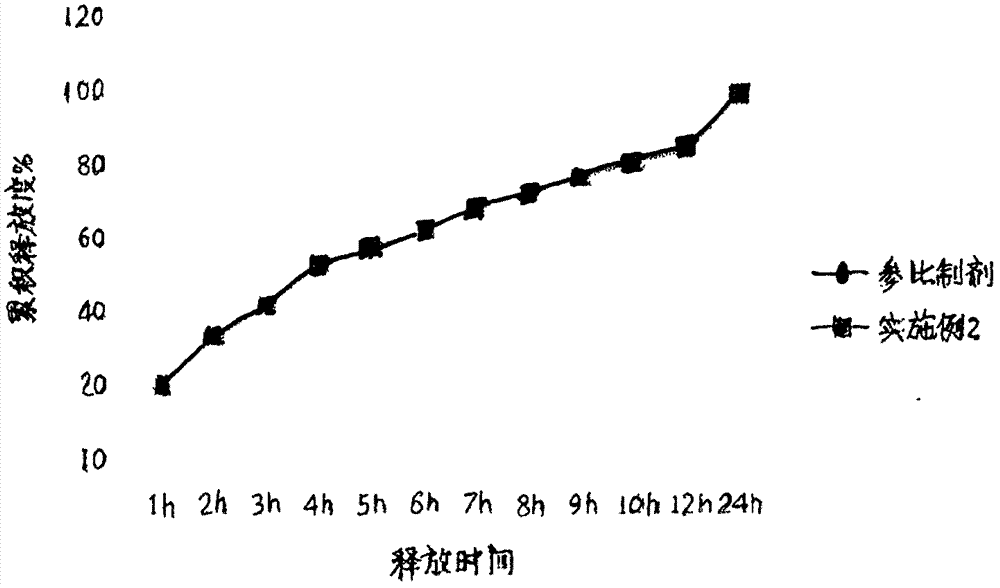
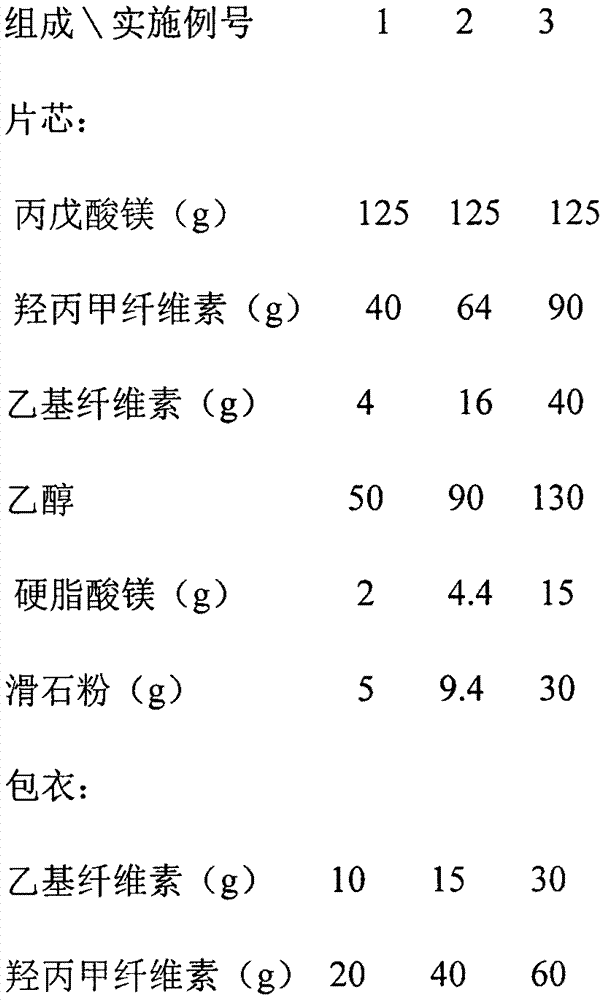
[0026] Example: 1000 tablets
[0027]
[0028]
[0029] Preparation method: crush magnesium valproate and pass through an 80-mesh sieve for later use; pass hypromellose, ethyl cellulose and magnesium stearate through a 100-mesh sieve for later use; Add an appropriate amount of ethanol to make a soft material, granulate through a 24-mesh sieve, then dry at a temperature of 55°C-65°C, sieve through a 24-mesh granule, and then add magnesium stearate, talc powder (or micro-powder silica gel) to mix After uniformity, measure the content, calculate the weight of the tablet, and press it into an oval tablet with a hardness of 40N-70N. Finally, the coating solution prepared from the above coating materials is used for coating.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com