Magnesium valproate sustained-release tablet and preparing method thereof
A technology of magnesium valproate and sustained-release tablets, which is used in pharmaceutical formulations, anhydride/acid/halide active ingredients, and pill delivery, etc., can solve the problems of packaging, transportation, storage inconvenience, low drug release stability, and gastrointestinal tract. The problem of high irritation, etc., to achieve stable therapeutic effect, simple and easy preparation method, and easy absorption.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A sustained-release magnesium valproate tablet is characterized in that: it is made of the following raw materials: 250 mg magnesium valproate, 150-245 mg hypromellose, and 5-20 mg lubricant.
[0026] The preferred weight ratio of the raw materials is: 250mg magnesium valproate, 140mg hypromellose, 10mg lubricant.
[0027] The hypromellose can be one or two of the following models: 75hd100cr hypromellose, 75hd4000cr hypromellose, 75hd15000cr hypromellose, 100000cr hypromellose.
[0028] The lubricant is one or both of magnesium stearate, talcum powder and silicon dioxide.
[0029] Its production method includes the following steps:
[0030] Step 1: material preparation: according to the above mass ratio, magnesium valproate is pulverized with a pulverizer, and passed through a 100-mesh sieve;
[0031] Step 2: Mixing: Weigh magnesium valproate and hypromellose according to the above mass ratio, put them into a three-dimensional mixer and mix for 30 minutes to make medi...
Embodiment 2
[0038] Indications:
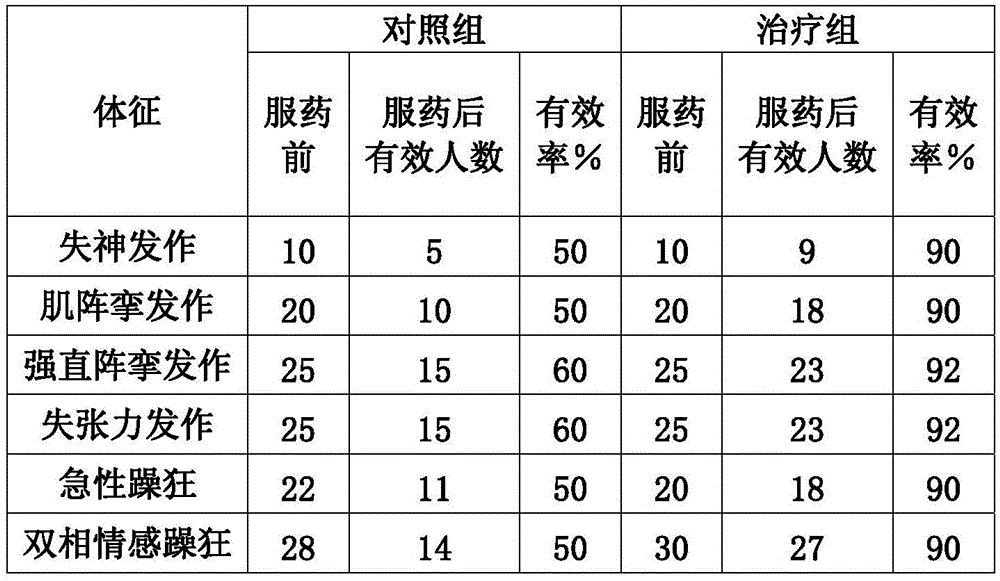
[0039] 1. Antiepileptic: for the treatment of generalized or partial epilepsy, especially the following types: absence seizures, myoclonic seizures, tonic-clonic seizures, atonic seizures and mixed seizures and partial epilepsy: simple or complex seizures; secondary generalized seizures; special types of syndromes (West, Lennox-Gastaut).
[0040] 2. Anti-mania: It is mainly used for the treatment of acute mania, bipolar mania and schizoaffective mania.
Embodiment 3
[0042] medicine interactions:
[0043] 1. Drinking alcohol can increase the sedative effect.
[0044] 2. When general anesthetics or central nervous system depressants are used together with valproic acid, the clinical effect of the former may be more obvious.
[0045] 3. Combined with anticoagulant drugs such as warfarin or heparin, and thrombolytic drugs, the risk of bleeding increases.
[0046] 4. When combined with aspirin or dipyridamole, the bleeding time is prolonged due to the reduction of platelet aggregation.
[0047] 5. When used in combination with phenobarbitals, the metabolism of the latter slows down and the blood concentration increases, thus increasing the sedative effect and causing drowsiness.
[0048] 6. Combined use with primidone can also cause an increase in blood concentration and lead to poisoning, and the dosage of primidone should be reduced if necessary.
[0049] 7. Combined with clonazepam to prevent and treat absence seizures, it has been repor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com