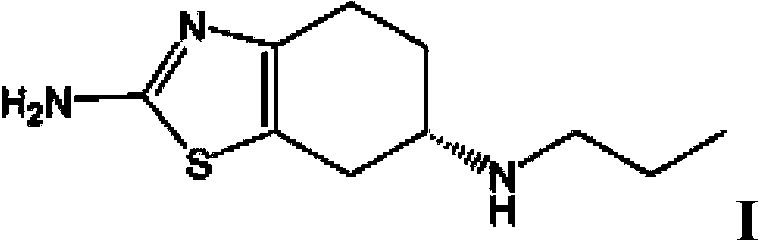
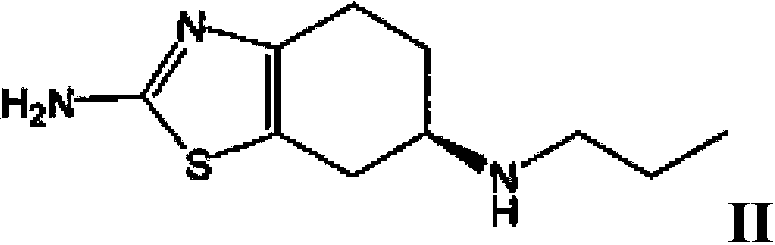
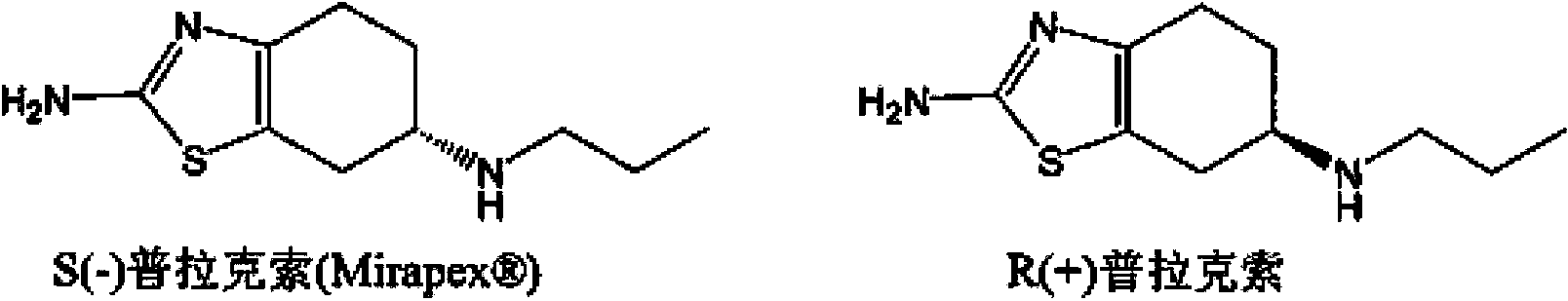Tetrahydrobenzothiazole derivative and preparation method thereof
A technology of benzothiazole and tetrahydro, which is applied in the field of tetrahydrobenzothiazole derivatives and its preparation, can solve the problems of unsuitability for industrial production, explosion of tetrahydrofuran, and very high requirements for equipment and workshops
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] (1) Preparation of (S)-(-)2-amino-6-propylamino-4,5,6,7-tetrahydrobenzothiazole
[0076] Add (-) 2-amino-6-propionylamino-4,5,6,7-tetrahydrobenzothiazole (227.33 g, 1 mol) into tetrahydrofuran (1362 ml), stir to dissolve, then add boron rapidly Sodium hydride (94.68g, 2.5mol) was stirred, then cooled to -5°C, and then slowly added dropwise at -5 to 0°C 2 (253.8g, 1mol) and tetrahydrofuran 725ml (I 2 The mass concentration of the solution is 35%). After dropping, slowly heat up to 35°C, keep the temperature for 8 hours, then cool to below 10°C, first add 45ml of tap water dropwise to avoid excessive reaction, and then add dropwise the mass fraction of 37% 946.8ml of hydrochloric acid, then slowly raise the temperature to 40°C, keep it for 30min, then recover tetrahydrofuran by vacuum distillation, after recovery, adjust the pH of the remaining solution to 12 with 30% sodium hydroxide solution, precipitate a large amount of solids, cool down to below 10°C , stirred fo...
Embodiment 1c
[0079] Example 1c: (R)-(+)2-amino-6-propylamino-4,5,6,7-tetrahydrobenzothiazole disalt for test Preparation of acid monohydrate
[0080] (R)-(+)2-amino-6-propylamino-4,5,6,7-tetrahydrobenzothiazole used in the present invention can refer to J.Med.Chem.1987,30,494- (+) 2-amino-6-propionylamino-4,5,6,7-tetrahydrobenzothiazole was prepared by the method provided in 498, and then prepared by referring to the above method. In another experiment, the present inventors prepared (R)-(+)2-amino-6-propylamino-4,5,6,7-tetrahydrobenzothiazole, and obtained (R)-(+) The chromatographic purity of 2-amino-6-propylamino-4,5,6,7-tetrahydrobenzothiazole dihydrochloride monohydrate is 99.62% (that is, (R)-(+)2-amino-6-propylamino- The molar ratio of 4,5,6,7-tetrahydrobenzothiazole to (S)-(-)2-amino-6-propylamino-4,5,6,7-tetrahydrobenzothiazole (peak area ratio ) is 262:1). It is clear to those skilled in the art that no matter how high the chromatographic purity of the (R)-(+)2-amino-6-pro...
Embodiment 2
[0081] Example 2: Preparation of (S)-(-)2-amino-6-propylamino-4,5,6,7-tetrahydrobenzothiazole dihydrochloride
[0082] Refer to the method of Example 1 of CN1834092A to obtain (S)-(-)2-amino-6-propylamino-4,5,6,7-tetrahydrobenzothiazole dihydrochloride. As determined by HPLC, (S)-(-)2-amino-6-propylamino-4,5,6,7-tetrahydrobenzothiazole and (R)-(+)2-amino-6- The peak area ratio (also known as molar ratio) of propylamino-4,5,6,7-tetrahydrobenzothiazole is 117:1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com