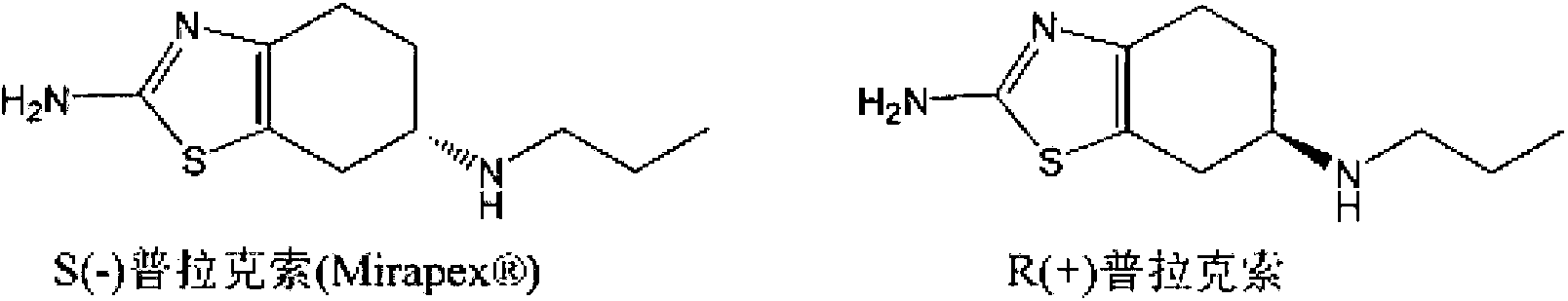Tetrahydrobenzothiazole derivate for treating nerve diseases
A benzothiazole and tetrahydro technology, applied in the field of tetrahydrobenzothiazole derivatives, can solve problems such as difficult industrial production, low boiling point of tetrahydrofuran, damage to reaction equipment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
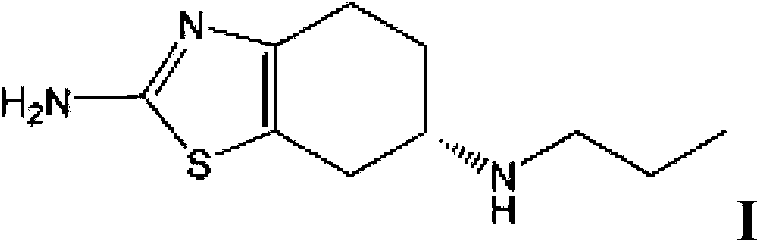
[0075] (1) Preparation of (S)-(-)2-amino-6-propylamino-4,5,6,7-tetrahydrobenzothiazole
[0076] Add (-) 2-amino-6-propionylamino-4,5,6,7-tetrahydrobenzothiazole (227.33 g, 1 mol) into tetrahydrofuran (1362 ml), stir to dissolve, then add boron rapidly Sodium hydride (94.68g, 2.5mol) was stirred, then cooled to -5°C, and then slowly added dropwise at -5 to 0°C 2 (253.8g, 1mol) and tetrahydrofuran 725ml (I 2 The mass concentration of the solution is 35%). After dropping, slowly heat up to 35°C, keep the temperature for 8 hours, then cool to below 10°C, first add 45ml of tap water dropwise to avoid excessive reaction, and then add dropwise the mass fraction of 37% 946.8ml of hydrochloric acid, then slowly raise the temperature to 40°C, keep it for 30min, then recover tetrahydrofuran by vacuum distillation, after recovery, adjust the pH of the remaining solution to 12 with 30% sodium hydroxide solution, precipitate a large amount of solids, cool down to below 10°C , stirred fo...
Embodiment 1c
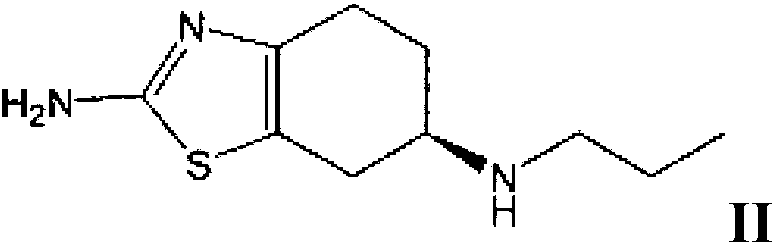
[0079] Example 1c: (R)-(+)2-amino-6-propylamino-4,5,6,7-tetrahydrobenzothiazole disalt for test Preparation of acid monohydrate
[0080] (R)-(+)2-amino-6-propylamino-4,5,6,7-tetrahydrobenzothiazole used in the present invention can refer to J.Med.Chem.1987,30,494- (+) 2-amino-6-propionylamino-4,5,6,7-tetrahydrobenzothiazole was prepared by the method provided in 498, and then prepared by referring to the above method. In another experiment, the present inventors prepared (R)-(+)2-amino-6-propylamino-4,5,6,7-tetrahydrobenzothiazole, and obtained (R)-(+) The chromatographic purity of 2-amino-6-propylamino-4,5,6,7-tetrahydrobenzothiazole dihydrochloride monohydrate is 99.62% (that is, (R)-(+)2-amino-6-propylamino- The molar ratio of 4,5,6,7-tetrahydrobenzothiazole to (S)-(-)2-amino-6-propylamino-4,5,6,7-tetrahydrobenzothiazole (peak area ratio ) is 262:1). It is clear to those skilled in the art that no matter how high the chromatographic purity of the (R)-(+)2-amino-6-pro...
Embodiment 2
[0081] Example 2: Preparation of (S)-(-)2-amino-6-propylamino-4,5,6,7-tetrahydrobenzothiazole dihydrochloride
[0082] Refer to the method of Example 1 of CN1834092A to obtain (S)-(-)2-amino-6-propylamino-4,5,6,7-tetrahydrobenzothiazole dihydrochloride. As determined by HPLC, (S)-(-)2-amino-6-propylamino-4,5,6,7-tetrahydrobenzothiazole and (R)-(+)2-amino-6- The peak area ratio (also known as molar ratio) of propylamino-4,5,6,7-tetrahydrobenzothiazole is 117:1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com