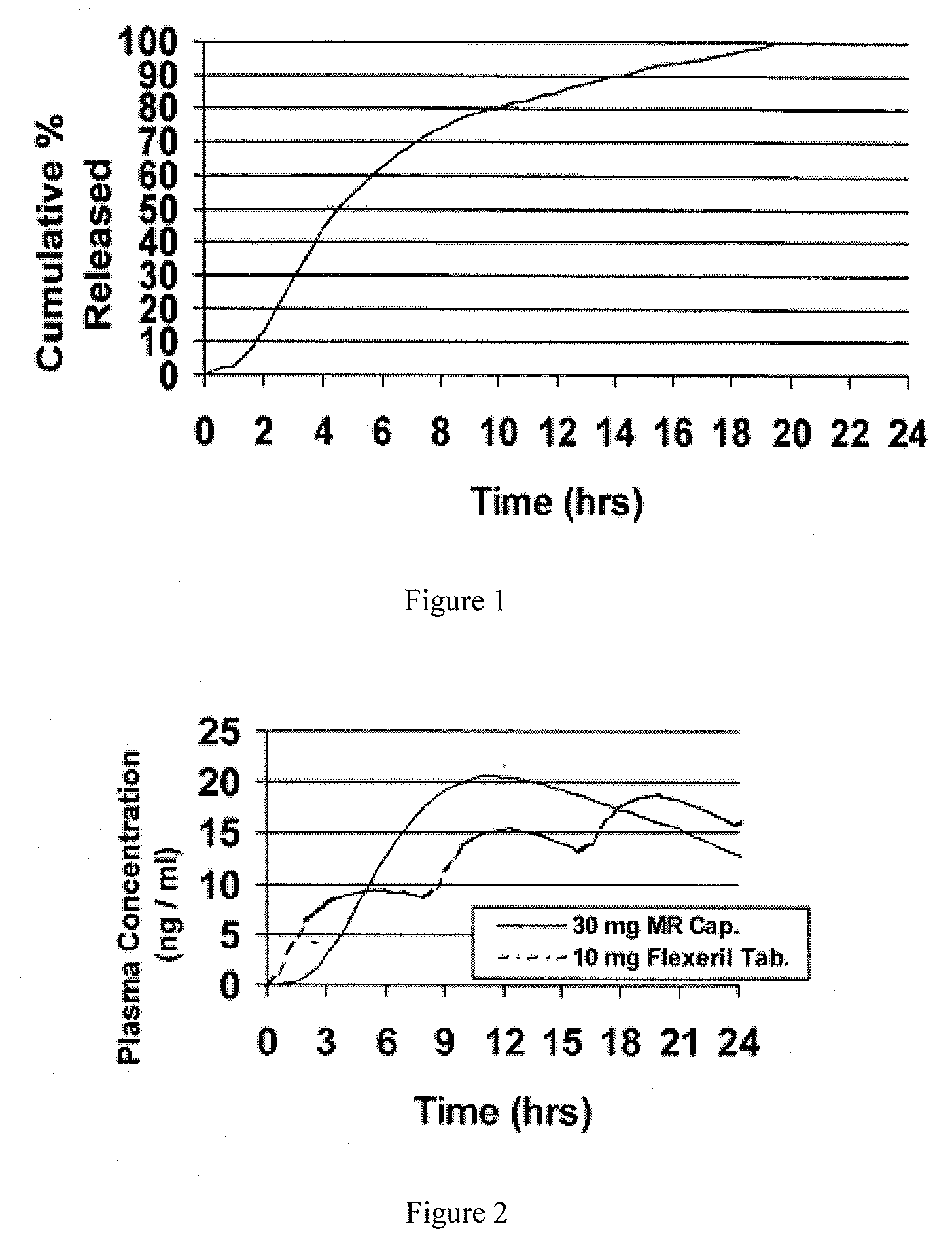
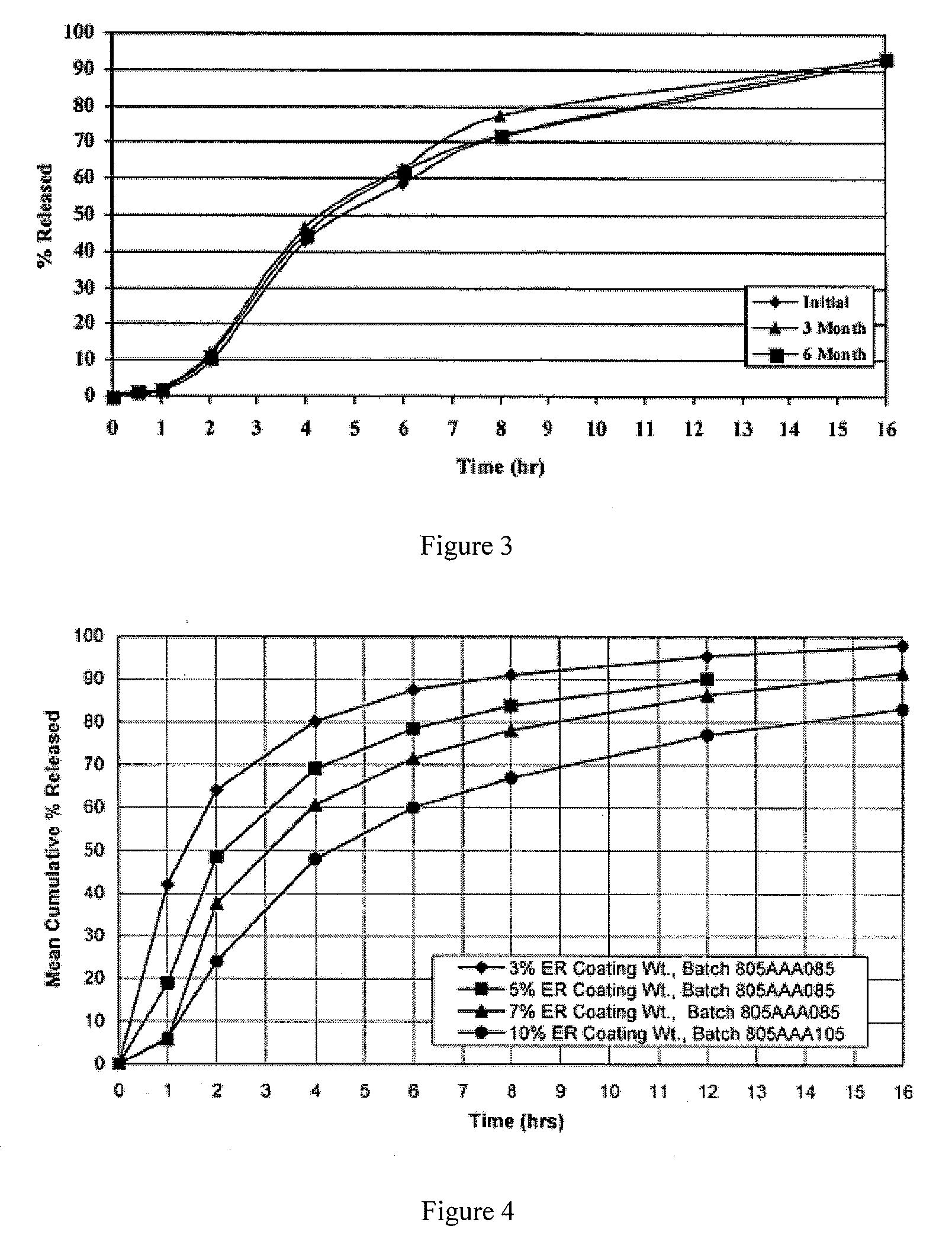
Patents
Literature
31 results about "Cyclobenzaprine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
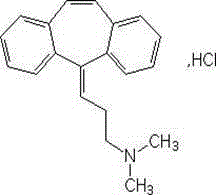
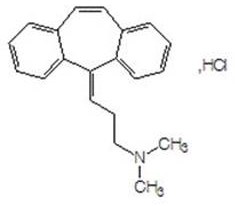
Cyclobenzaprine is used short-term to treat muscle spasms. It is usually used along with rest and physical therapy.
Pharmaceutical compositions comprising RET inhibitors and methods for the treatment of cancer
InactiveUS8629135B2Reduce transferGrowth inhibitionBiocideOrganic compound preparationCancer preventionAutophosphorylation
Owner:SINGH VINAY K +2
Pharmaceutical Compositions Comprising RET Inhibitors and Methods for the Treatment of Cancer
InactiveUS20110201598A1Inhibit tumor growthReduce transferBiocideOrganic compound preparationCancer preventionPancreas Cancers
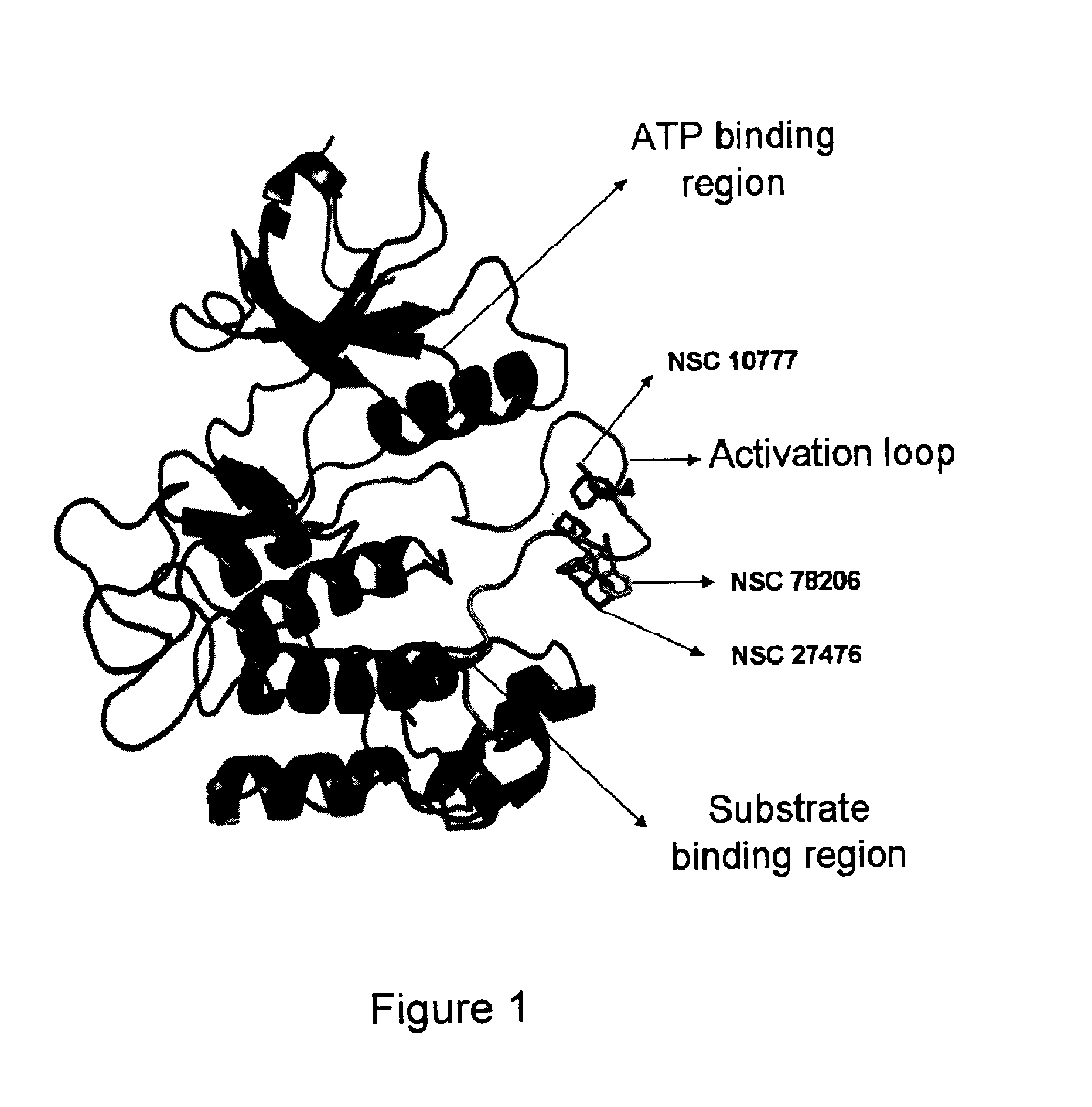
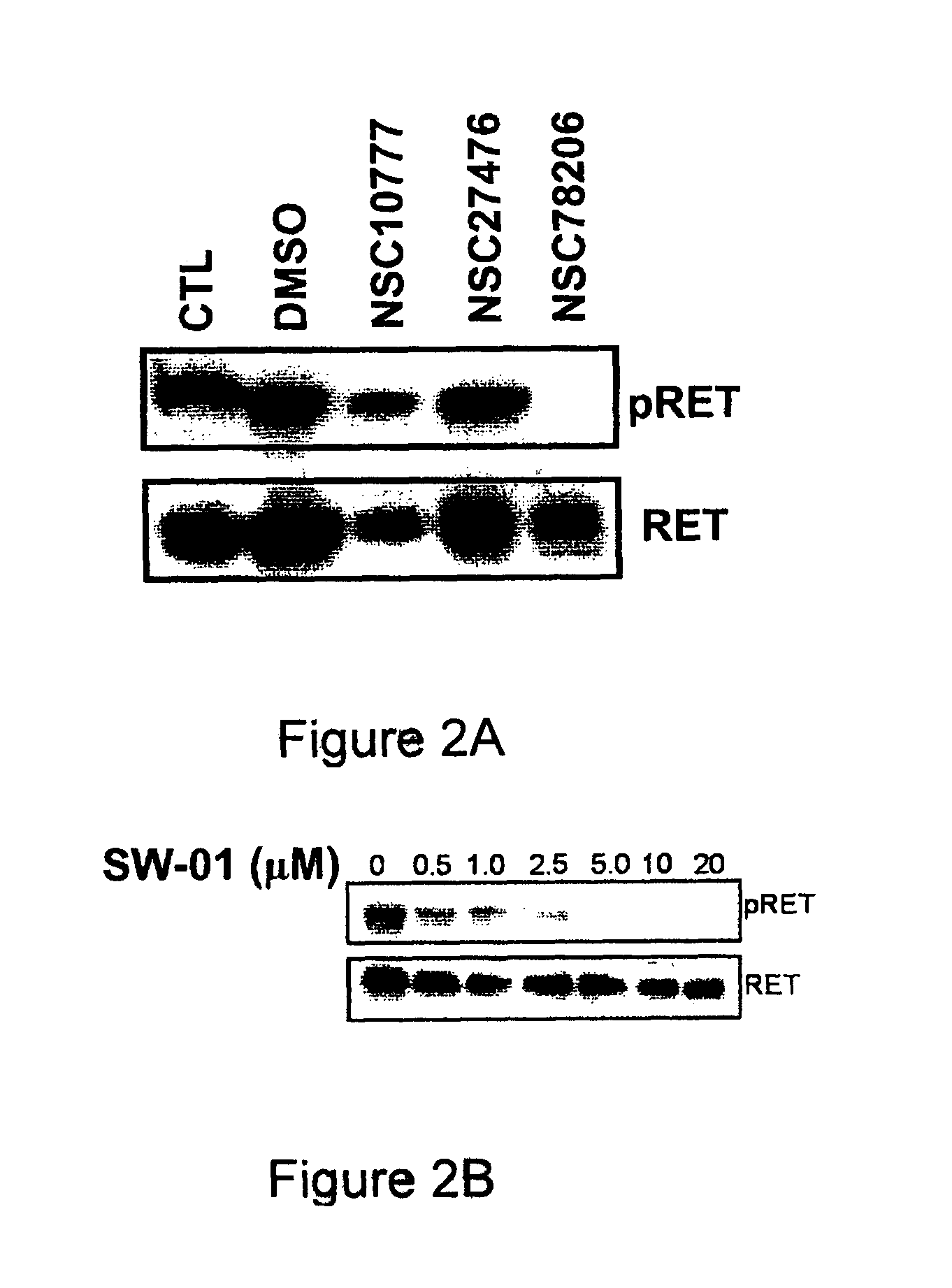
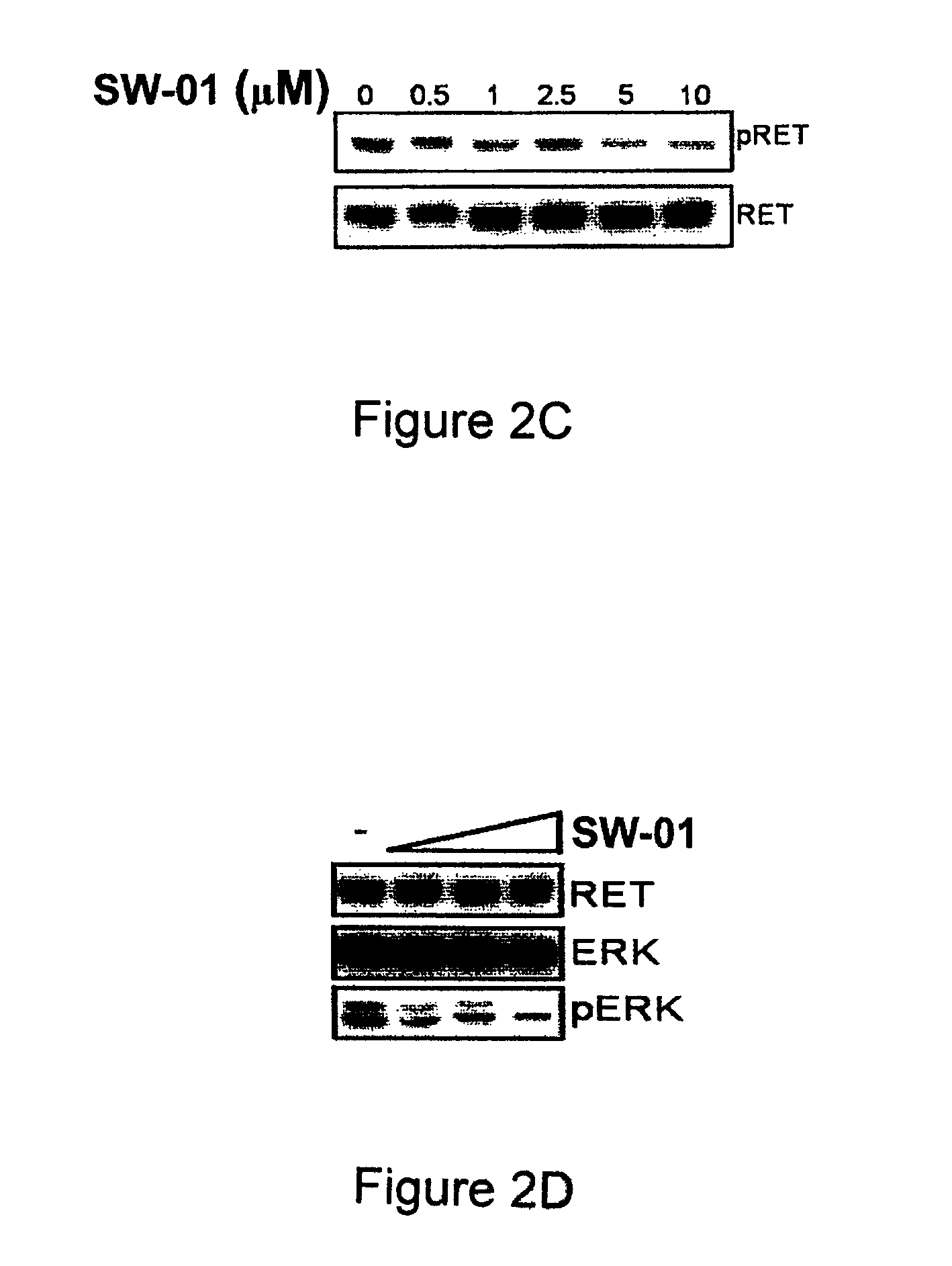
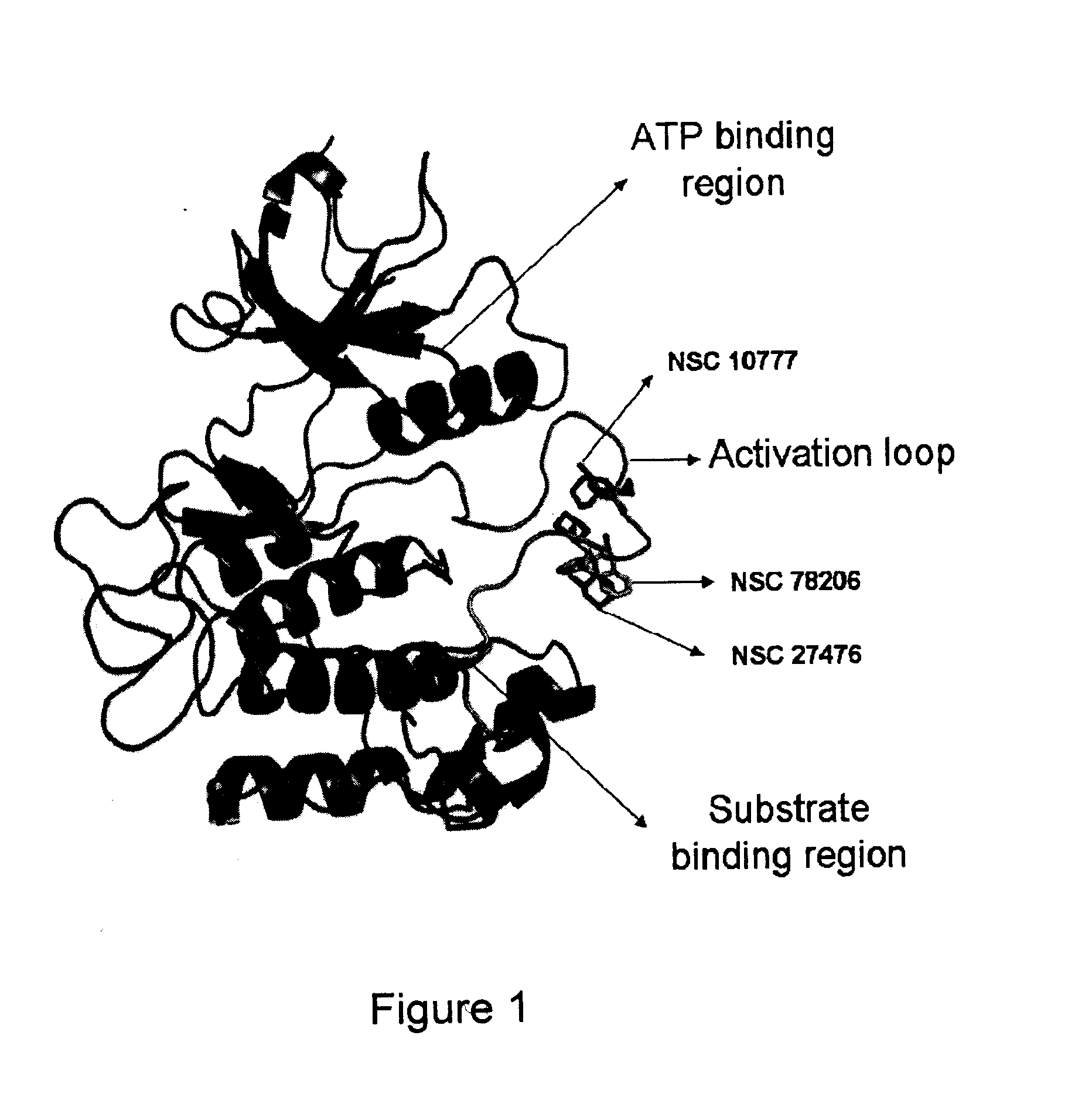
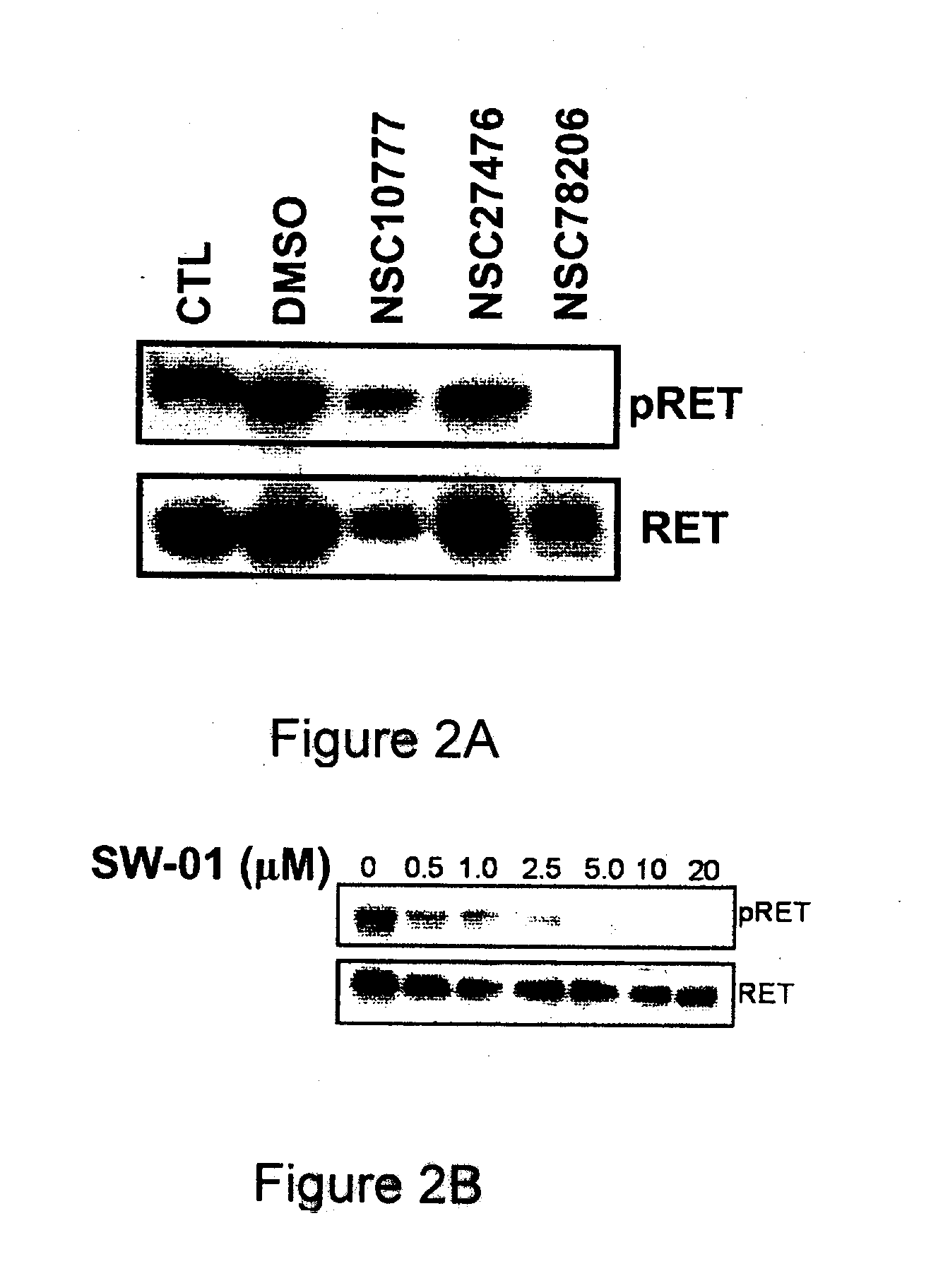
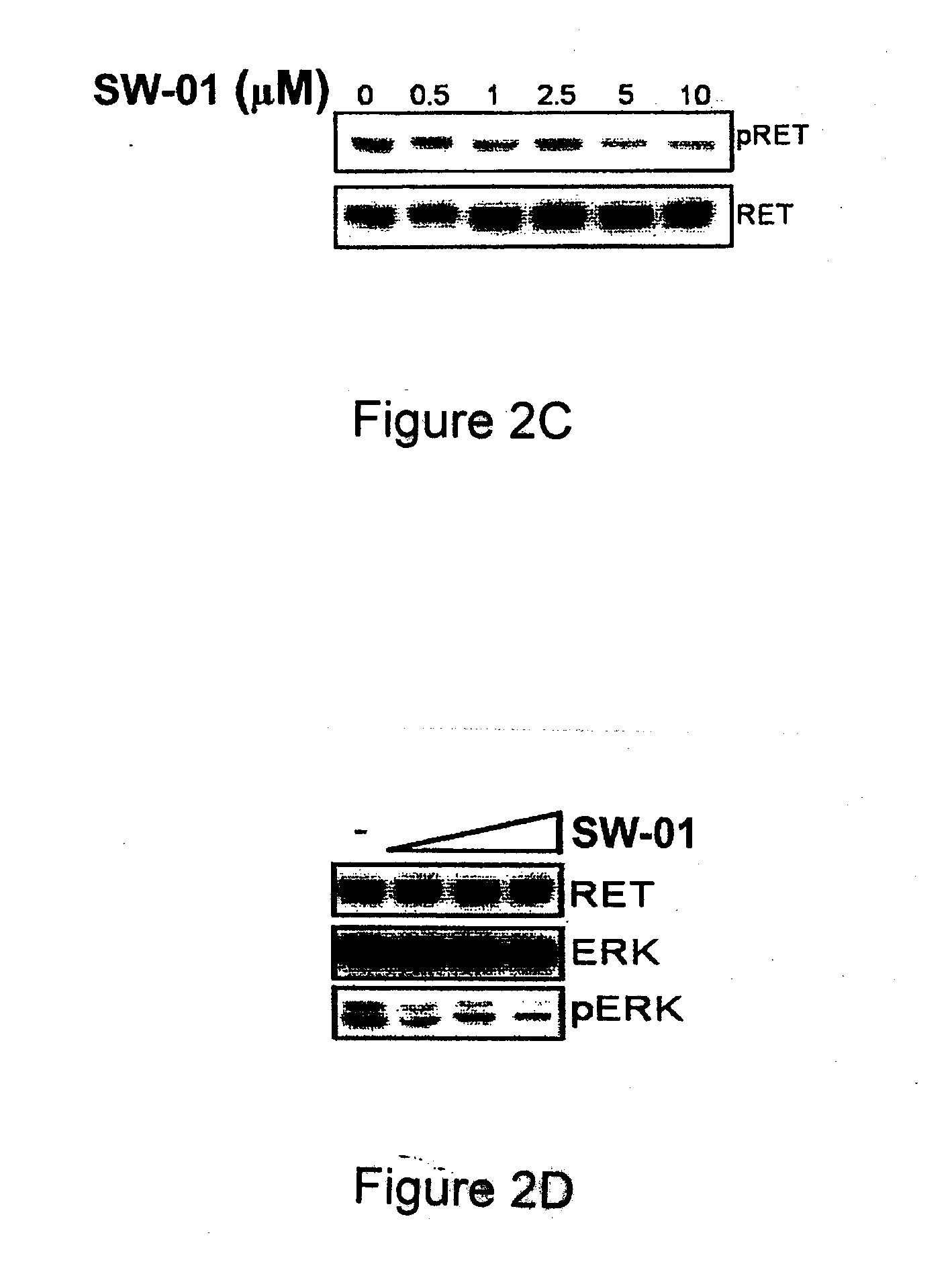
A class of compounds useful in pharmaceutical compositions and methods for treating or preventing cancer is described. The compounds' ability to inhibit RET kinase is quantified, i.e., their respective RET IC50 and EC50 values are described. One such compound, known as cyclobenzaprine and herein as SW-01, has been identified as RET-specific with an IC50 of 300 nM. SW-01 inhibits RET autophosphorylation and blocks the growth and transformation of thyroid cancer cell lines. It has been further tested in pancreatic cancer, breast cancer, and SCLC cell lines. The compounds show utility for inhibition of survival and proliferation of tumour cells.
Owner:SINGH VINAY K +2
Nutrigenomics methods and compositions
The present invention provides a proprietary compositions and systems to modulate genetic and metabolomic contributing factors affecting disease diagnosis, stratification, and prognosis, as well as the metabolism, efficacy and / or toxicity associated with specific vitamins, minerals, herbal supplements, homeopathic ingredients, and other ingredients for the purposes of customizing a subject's nutritional supplement formulation to optimize specific health outcomes. Specific to this invention the utilization of certain known polymorphic genes associated with Substance Use Disorder (SUD) are analyzed to target certain genetic anomalies that lead to a high risk and predisposition to SUD. The genotypic patterns are then utilized to provide certain nutritional customized solutions especially related to the attenuation of aberrant abuse of physician prescribed narcotic pain medication across all pain conditions. A priority GENOPROFILE is measured and directs the customization of a subsequent nutraceutical to act as a therapeutic modality. Specifically the treatment includes slow attenuation of the pain medication by incorporating orals (shakes, liquid beverages, pills, tablets, troche, ointments etc.), Intramuscular, Intravenous, intra-rectal and any form necessary to deliver a sufficient amount of an anti-craving and anti-stress nutraceutical. Moreover, the invention includes examples of novel analgesic ointments coupling Synaptamine and such analgesic and other anesthetic compounds including but not limited to Gabapentin, Ketamine, Baclofen, Ketoprofen, Amitriptyline, Lidocaine, Cyclobenzapine, Diclofenac, Menthol, Camphor and Capsaicin. The GENOPROFILE will be used to determine pain sensitivity Intolerance.
Owner:BLUM KENNETH +3
Preparation of controlled release skeletal muscle relaxant dosage forms
Owner:ADARE PHARM INC
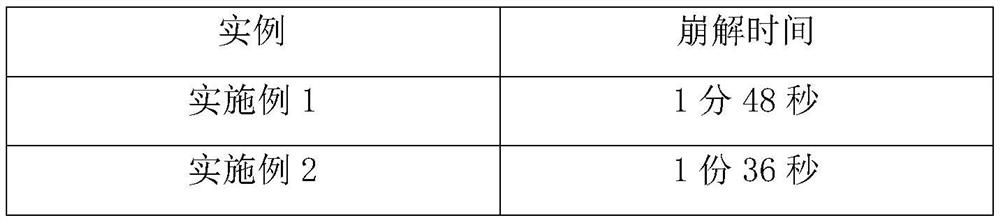
Cyclobenzaprine hydrochloride sustained release tablets and preparation method thereof
InactiveCN104352474AReduce absorption rateImprove stabilityOrganic active ingredientsMuscular disorderMuscle spasmTreatment pain
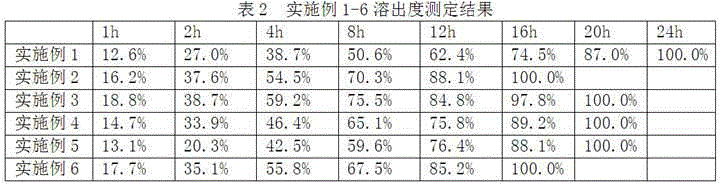
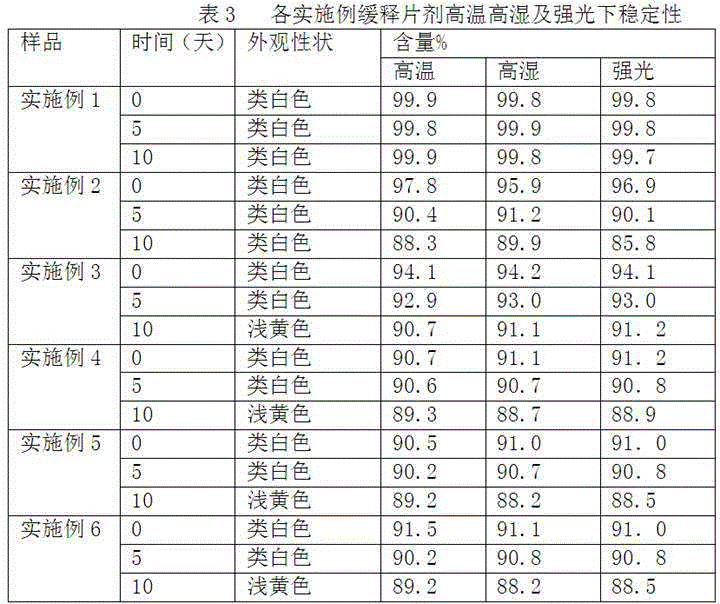
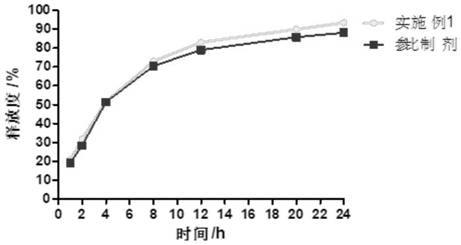
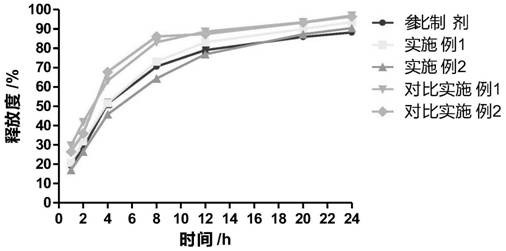
The invention provides cyclobenzaprine hydrochloride sustained release tablets. The cyclobenzaprine hydrochloride sustained release tablets are prepared from 15 parts of cyclobenzaprine hydrochloride, 75-80 parts of sustained release skeleton material and 5-10 parts of lubricating agent by weight. A preparation method of the cyclobenzaprine hydrochloride sustained release tablets comprises the steps of material preparing, blending, granulating, blending, tabletting and aluminium-plastic packaging. The cyclobenzaprine hydrochloride sustained release tablets are adjuvant medicines for treating painful local muscle spasm and have the beneficial effects that the novel sustained release preparations are adopted; sustained release refers to reducing the medicine release rates of medicines from the dosage forms and reducing the absorption rates of the medicines into bodies, thus achieving more stable treatment effects; compared with oral liquids, the cyclobenzaprine hydrochloride sustained release tablets have the advantages of good medicine stability, convenience in packaging, transportation and storage, and the like. The preparation method is simple and practicable and is suitable for industrial production.
Owner:HARBIN SHENGJI PHARMA
Cyclobenzaprine hydrochloride sustained release pellets and preparation method thereof
InactiveCN104473908AReduce absorption rateStable absorptionOrganic active ingredientsAntipyreticSustained release pelletsIrritation
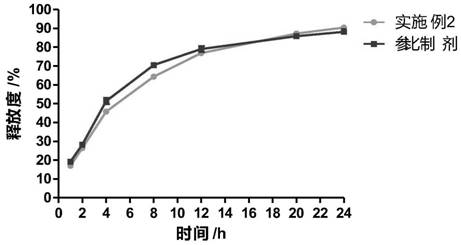
The invention discloses cyclobenzaprine hydrochloride sustained release pellets and a preparation method thereof. The cyclobenzaprine hydrochloride sustained release pellets comprise coating layers and medicine-containing pellets, wherein the medicine-containing pellets are externally coated with the coating layers; the coating layers comprise Eudragit NE30D, talcum powder and sodium dodecyl sulfate or polyethylene glycol; the medicine-containing pellets comprise cyclobenzaprine hydrochloride, hollow pellet cores, filling agents, lubricating agents and adhesives. The cyclobenzaprine hydrochloride sustained release pellets and the preparation method have the beneficial effects that the cyclobenzaprine hydrochloride sustained release pellets are mainly used as adjuvant medicines for treating painful local muscle spasms, adopt more novel sustained release preparations and pellet preparations and have the characteristics that the distribution areas of the medicines on the surfaces of the gastrointestinal tracts are enlarged, so that the irritation can be reduced, the bioavailability can be improved, and meanwhile, the medicines are not affected by the factor of gastric emptying, are uniform in internal absorption and have small individual difference; the technological advantages of the medicines are further enhanced by simultaneously applying the two types of advanced technologies; compared with oral liquids, the cyclobenzaprine hydrochloride sustained release pellets have the advantages of good medicine stability, convenience in packaging, transportation and storage, and the like; the preparation method is simple and practicable and is suitable for industrial production.
Owner:HARBIN SHENGJI PHARMA
Preparation of controlled release skeletal muscle relaxant dosage forms
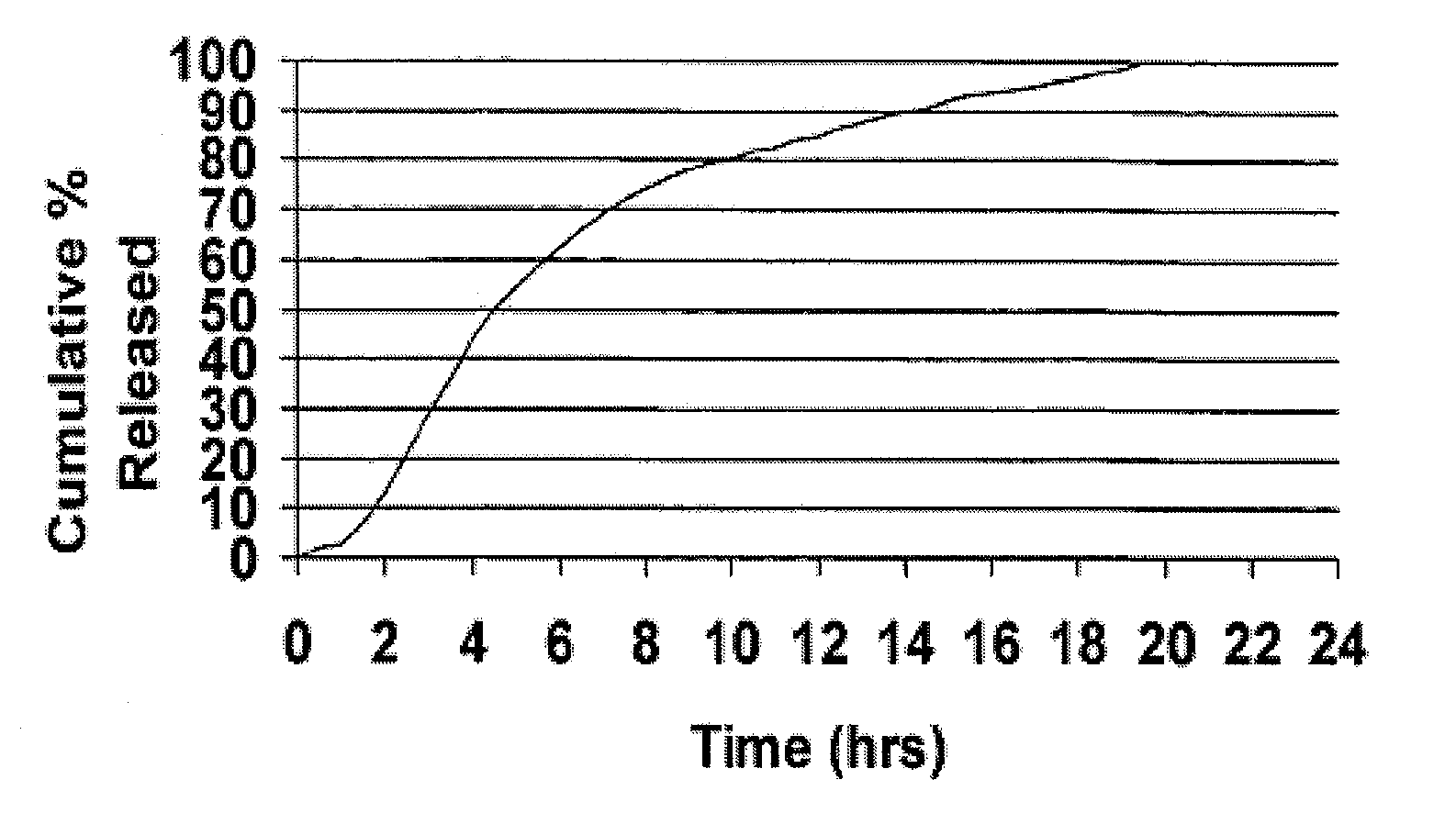
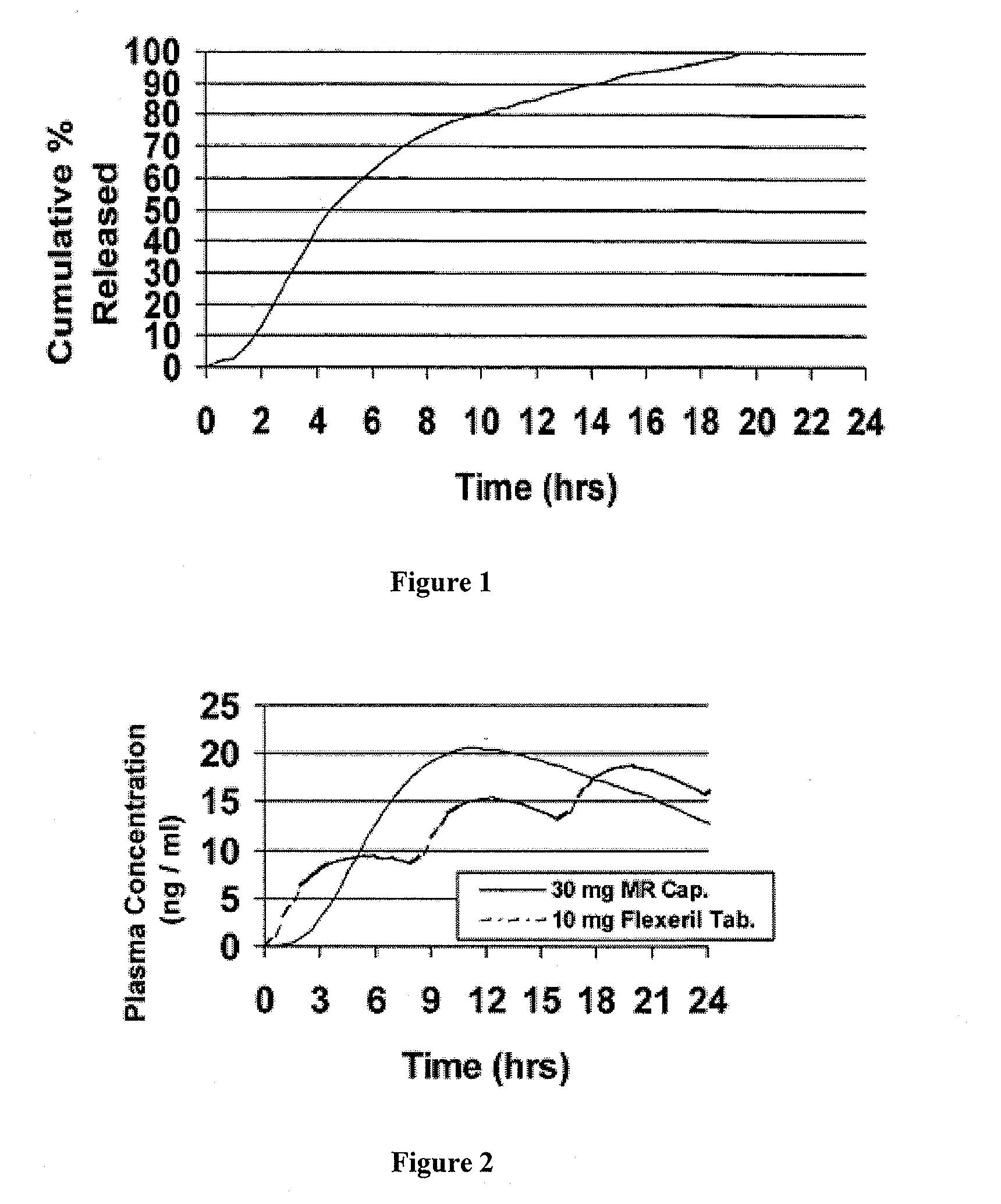
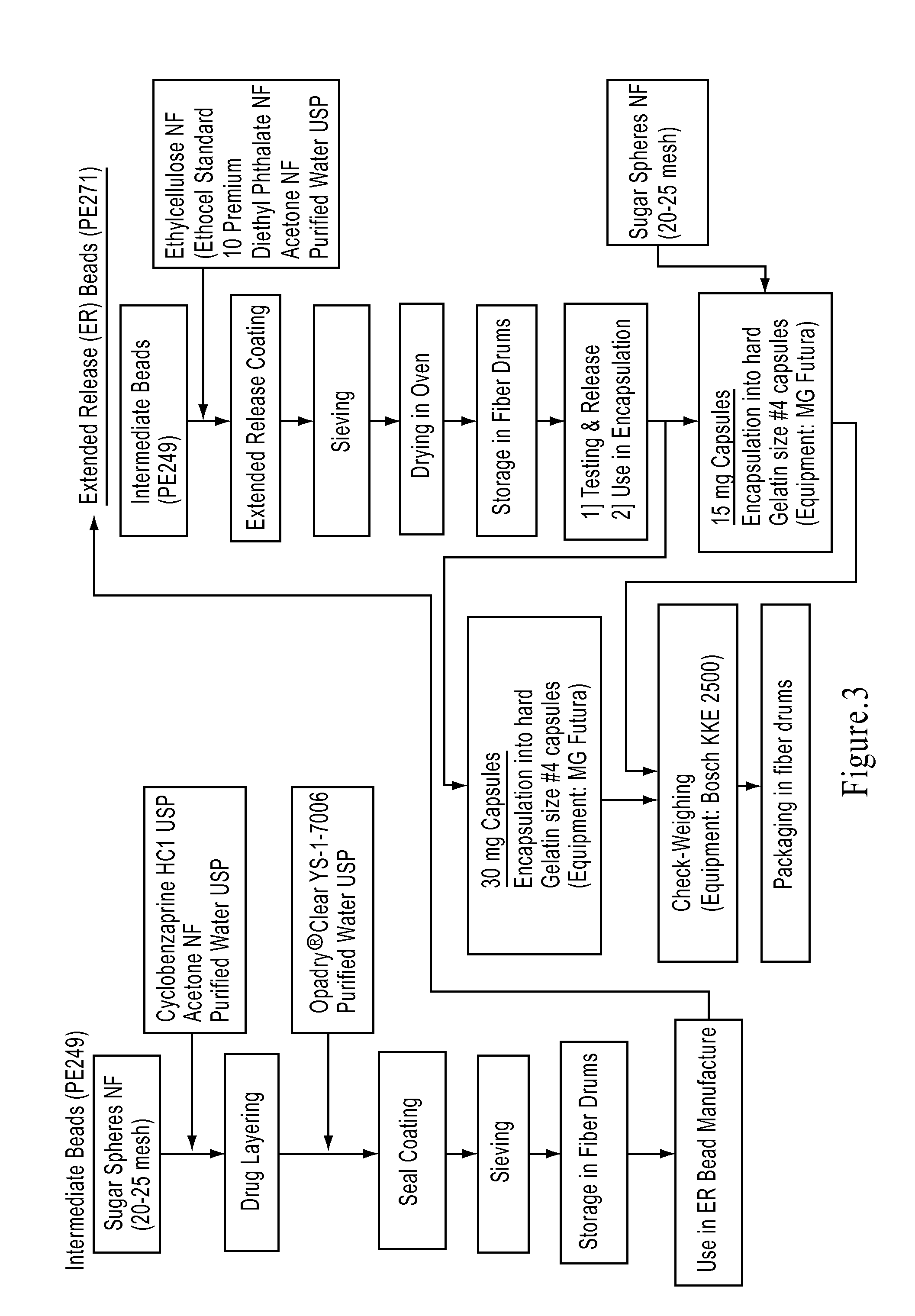
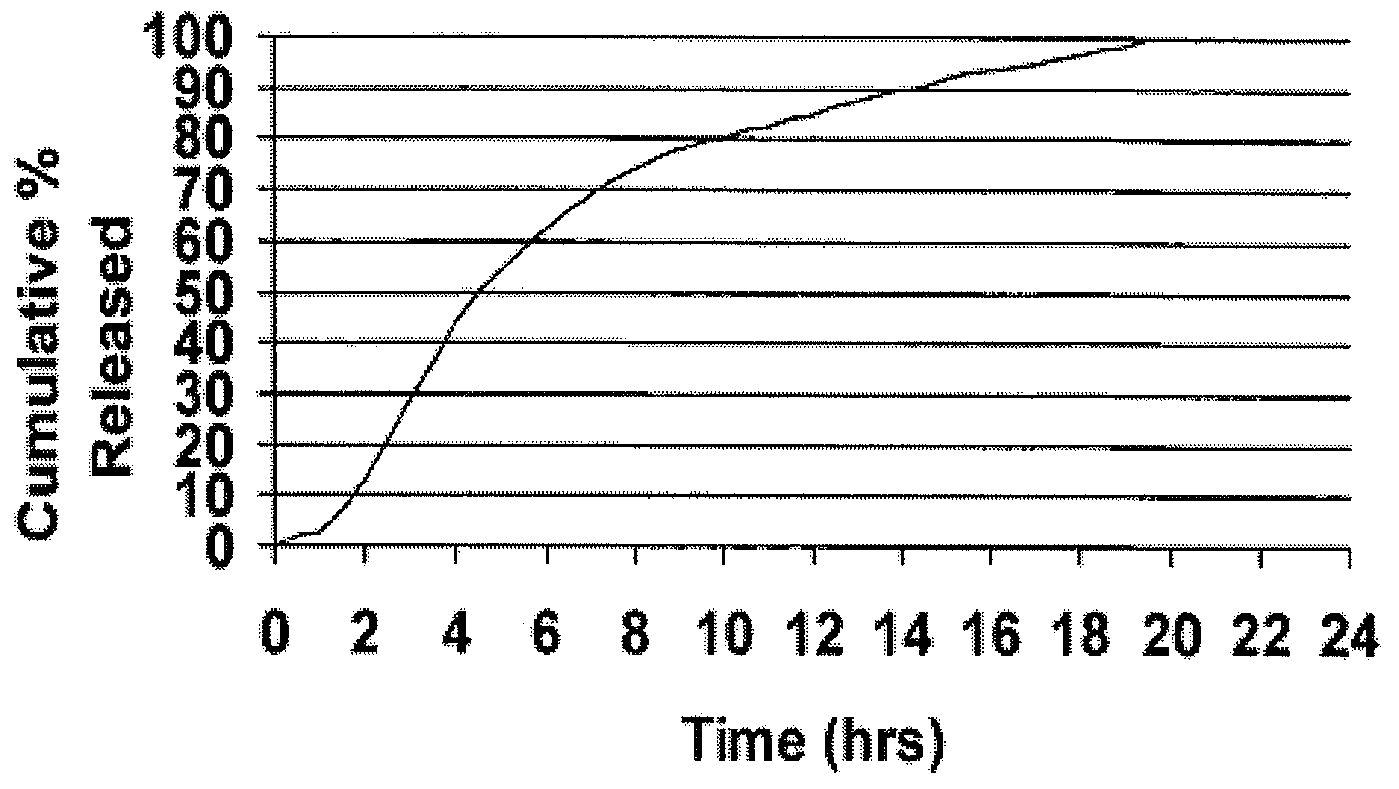
The present invention is directed to a method of preparing an extended release pharmaceutical composition comprising cyclobenzaprine, comprising coating inert particles with a cyclobenzaprine-containing the drug layering composition and a seal coating composition to form IR beads, then coating the IR beads with an extended release coating to form ER beads.
Owner:ADARE PHARM INC
Extended release pharmaceutical compositions
ActiveUS20120064164A1Reduce morbidityBiocideOrganic active ingredientsSkeletal muscleBuccal administration
The present invention provides extended release pharmaceutical compositions structured for once a day administration comprising skeletal muscle relaxant such as cyclobenzaprine or its pharmaceutically acceptable salt thereof that extends the release of the drug under in-vitro conditions for at least 8 to 12 hours. The invention also provides process for the preparation of such structured compositions.
Owner:INVENTIA HEALTHCARE LTD
Extended release tablet of cyclobenzaprine
InactiveUS20180116967A1Reduce adverse effectsEasy and cost-effective to manufactureOrganic active ingredientsMuscular disorderExtended release tabletsLactose
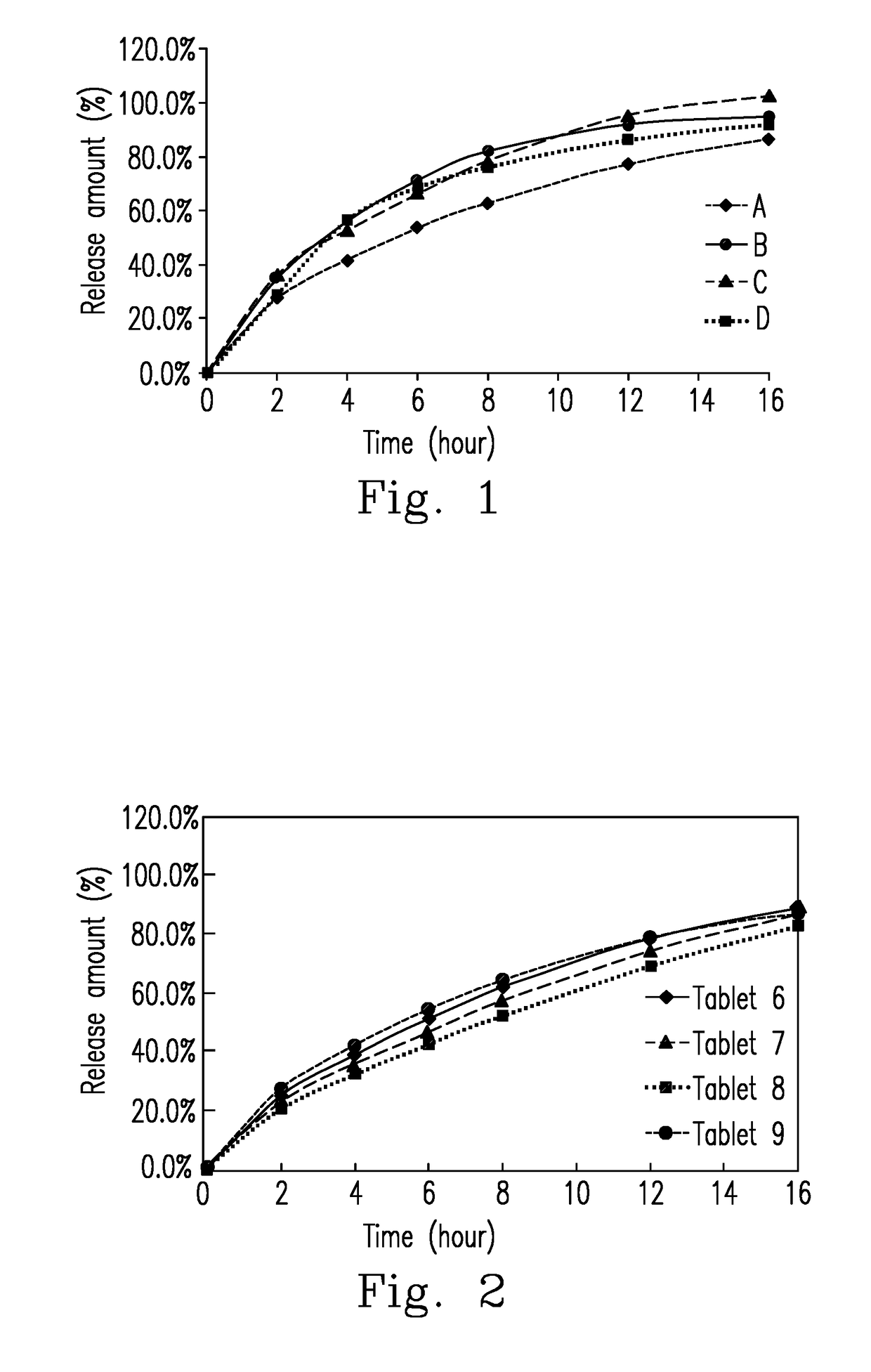
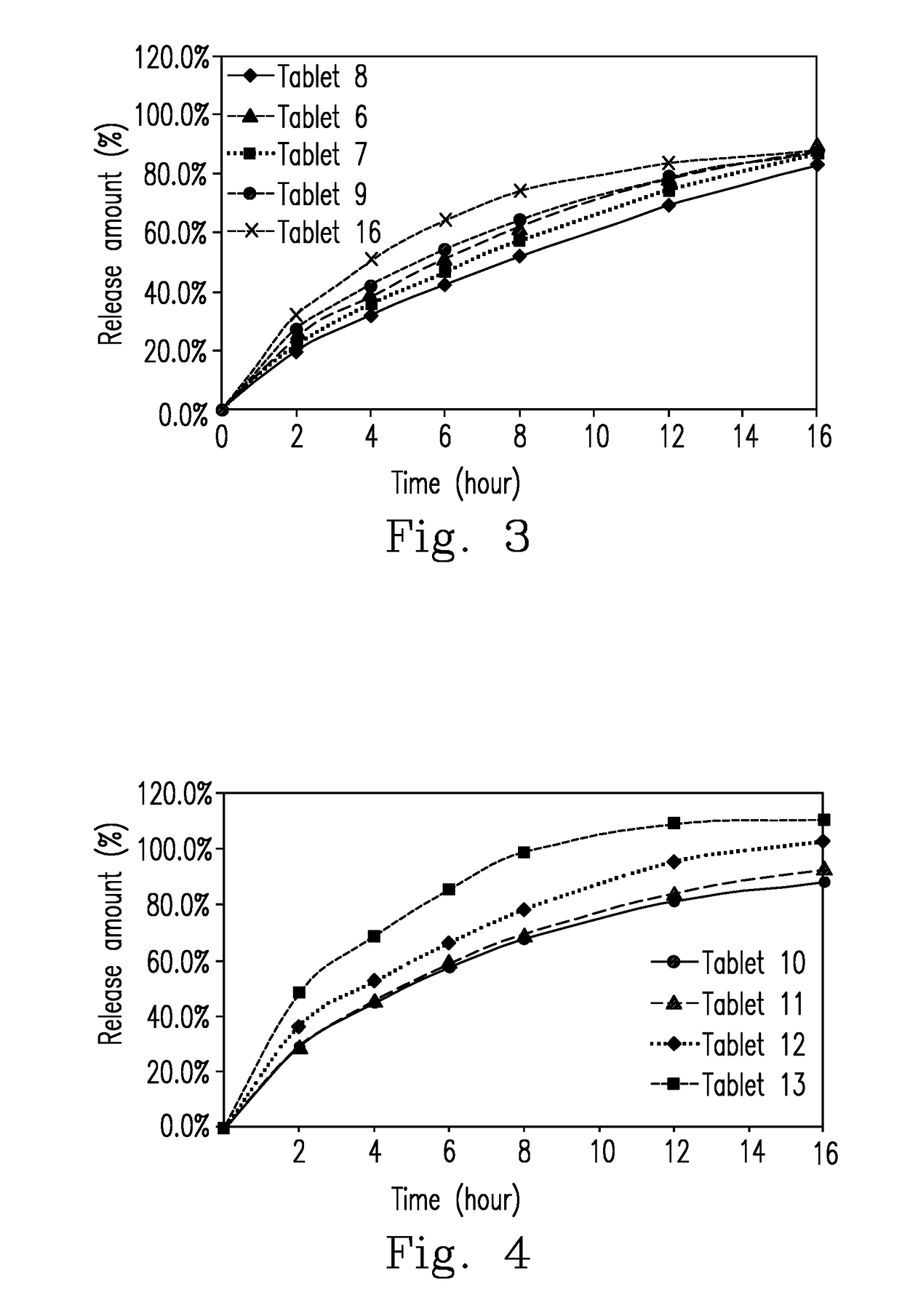
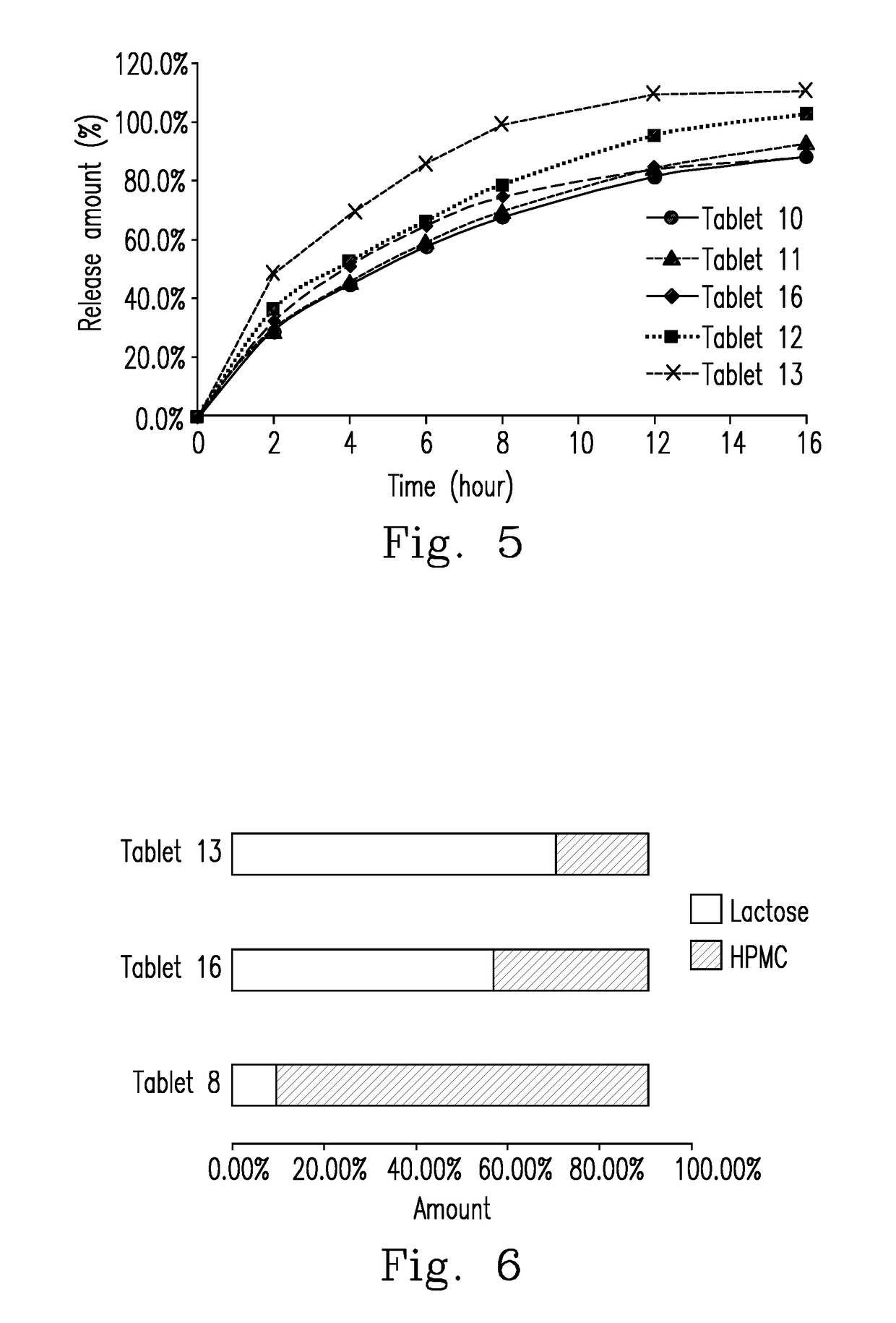
A directly compressed extended release cyclobenzaprine tablet and method for making the tablet that includes homogenously mixing: (i) cyclobenzaprine, (ii) a filler selected from the group consisting of lactose, spray-dried lactose, mannitol, and combinations thereof; and (iii) a glidant selected from the group consisting of silica, peptized silica, and combinations thereof; and (iv) hydroxypropyl methylcellulose (HPMC) to provide a first mixture; homogenously mixing a lubricant with the first mixture to provide a second mixture; and directly compressing the second mixture into a tablet, having a ratio of filler to matrix forming polymer ranges from 1.66 to 2.07.
Owner:SYNMOSA BIOPHARMA CORP
Cyclobenzaprine hydrochloride sustained-release tablet
ActiveCN105395507ASmooth releaseImprove complianceOrganic active ingredientsAntipyreticPatient complianceMuscle spasm
The invention provides a preparation method of a cyclobenzaprine hydrochloride sustained-release tablet, and belongs to the technical field of a medicine. The cyclobenzaprine hydrochloride sustained-release tablet disclosed by the invention consists of cyclobenzaprine hydrochloride, a sustained-release material, a pore-foaming agent, a filling agent, a lubricating agent and distilled water. The cyclobenzaprine hydrochloride, as a major ingredient, has effects of relieving muscle spasm as well as accompanied severe pain in skeletal muscle and the like; and the provided sustained-release preparation is safe and effective, stable in quality, low in cost and low in administration efficiency, and the sustained-release preparation is capable of enhancing patient compliance and is capable of stably stopping pain and relieving spasm.
Owner:CP PHARMA QINGDAO CO LTD
Cyclobenzaprine treatment for agitation, psychosis and cognitive decline in dementia and neurodegenerative conditions
Compositions comprising cyclobenzaprine, and methods for the treatment or prevention of agitation, psychosis and / or cognitive decline and associated symptoms thereof in dementia and other neurodegenerative conditions.
Owner:TONIX PHARMA HLDG LTD
Method for detecting cyclobenzaprine hydrochloride sustained-release capsules
InactiveCN111707764AGood linear relationshipImprove accuracyComponent separationPharmaceutical drugBiochemistry
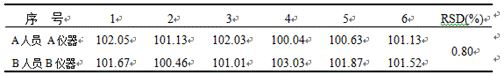
The invention relates to a detection method of cyclobenzaprine hydrochloride sustained-release capsules, and belongs to the field of pharmaceutical analysis. According to the detection method of cyclobenzaprine hydrochloride sustained-release capsules, cyclobenzaprine hydrochloride is detected through an HPLC method. According to the detection method disclosed by the invention, the cyclobenzaprinehydrochloride is good in linear relationship, good in accuracy and precision, strong in specificity and high in stability. The detection method has good reproducibility, can meet the detection requirements of cyclobenzaprine hydrochloride bulk drugs, and can be used for quality control of cyclobenzaprine hydrochloride sustained-release capsules.
Owner:CP PHARMA QINGDAO CO LTD
Analogs of cyclobenzaprine and amitryptilene
Owner:TONIX PHARMA HLDG LTD
Pharmaceutical Composition Comprising Cyclobenzaprine and Aceclofenac in Association
The present invention relates to an association of active ingredients. More specifically: to an association of cyclobenzaprine and aceclofenac. Additionally, the present invention is also related to the use of aceclofenac and cyclobenzaprine, in association for the preparation of a medicine useful in the treatment of painful muscular diseases, as well as to a method of treatment of painful muscular diseases using an association of aceclofenac and cyclobenzaprine.
Owner:BIOLAB SANUS FARMACEUTICA LTD +1
Method for detecting content of chloride ions in cyclobenzaprine hydrochloride
ActiveCN113687016AImprove solubilityEliminate distractionsChemical analysis using titrationMaterial electrochemical variablesActive agentTest sample
The invention provides a method for detecting the content of chloride ions in cyclobenzaprine hydrochloride. The method for detecting the content of chloride ions in cyclobenzaprine hydrochloride comprises the following steps: (1) preparing a sample to be tested: mixing cyclobenzaprine hydrochloride, nitric acid, a nonionic surfactant and water to obtain a sample solution to be tested; and (2) potentiometric titration analysis: titrating the sample solution to be tested by adopting a silver nitrate solution, and calculating to obtain the content of chloride ions. The test sample is analyzed by adopting a potentiometric titration method, and a titration end point is determined through potential change. The method has the advantages of being high in accuracy, rapid in analysis, high in anti-interference performance and high in sensitivity.
Owner:NOVAST LABORATORIES (CHINA) LTD
Cyclobenzaprine treatment for sexual dysfunction
The present disclosure provides a pharmaceutical composition comprising therapeutically effective amounts of cyclobenzaprine and one or more agents, and methods of treating and / or preventing sexual dysfunction.
Owner:TONIX PHARMA INC
Cyclobenzaprine hydrochloride sustained-release capsule and preparation method thereof
ActiveCN114533699ASustained release effect is stableSimple preparation processOrganic active ingredientsAntipyreticCyclobenzaprine hclHypromellose
Owner:NOVAST LABORATORIES (CHINA) LTD
A kind of cyclobenzaprine hydrochloride sustained-release preparation
ActiveCN109248153BIdeal release speedAddressing Medication Compliance IssuesOrganic active ingredientsMuscular disorderCyclobenzaprine hclBULK ACTIVE INGREDIENT
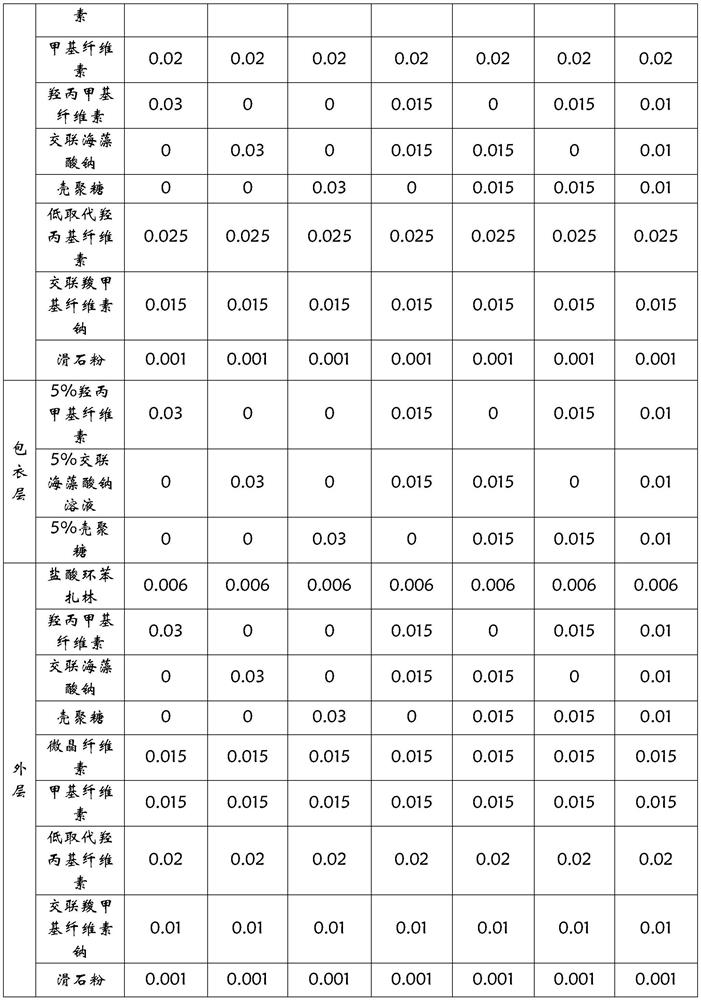
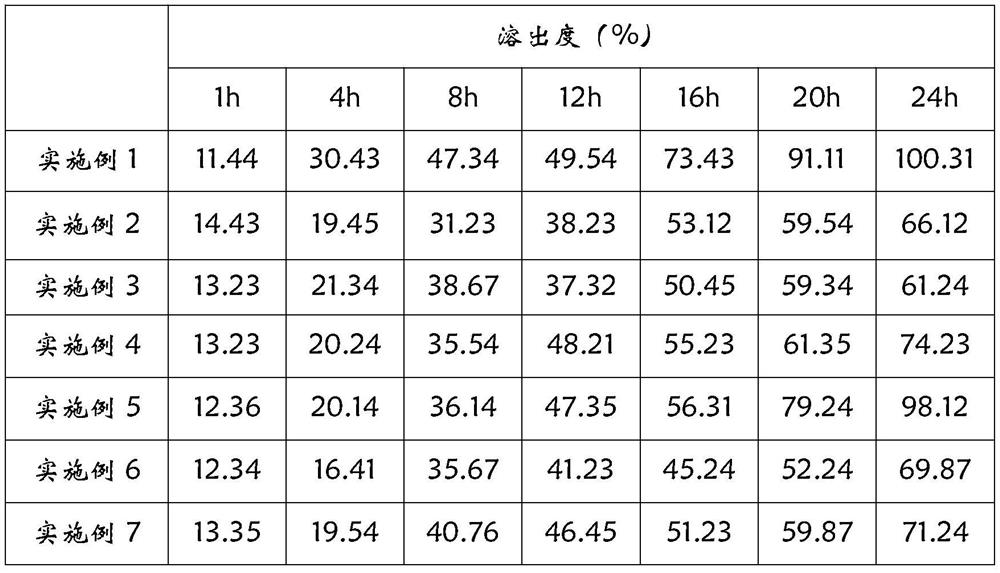
The invention relates to a sustained-release preparation of cyclobenzaprine hydrochloride, which belongs to the field of pharmaceutical preparations. The invention provides a cyclobenzaprine hydrochloride coated chip, the inner chip is composed of active ingredient cyclobenzaprine hydrochloride, sustained release agent, diluent, adhesive, disintegrating agent and lubricant, and the middle layer is sustained release agent The composition of the coating layer and the outer layer is the same as that of the inner chip. The cyclobenzaprine package chip of the present invention adopts cross-linked sodium alginate and chitosan as sustained-release agents, the release speed is ideal, the release is gentle within 24 hours, and the release is basically completed within 24 hours. The influencing factors test, accelerated test and The test results of the long-term sample retention test at room temperature all show that its stability is very good. The cyclobenzaprine hydrochloride package chip of the present invention well solves the problem that the cyclobenzaprine hydrochloride is frequently used due to its short half-life in the body, thus affecting the drug compliance of patients.
Owner:CP PHARMA QINGDAO CO LTD
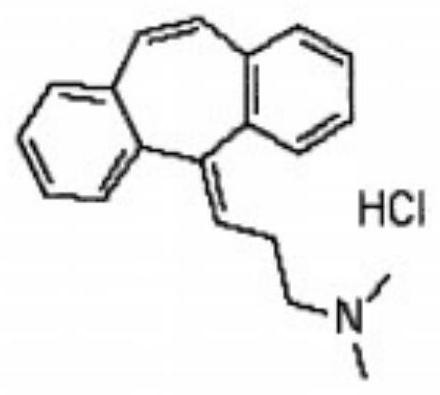
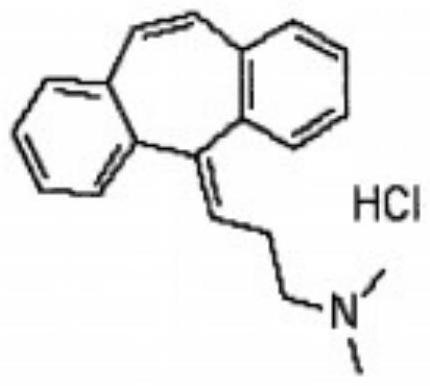
Refining method of cyclobenzaprine hydrochloride
InactiveCN111333518ANot easy to wrapHigh purityAmino compound purification/separationPhysical chemistryOrganosolv
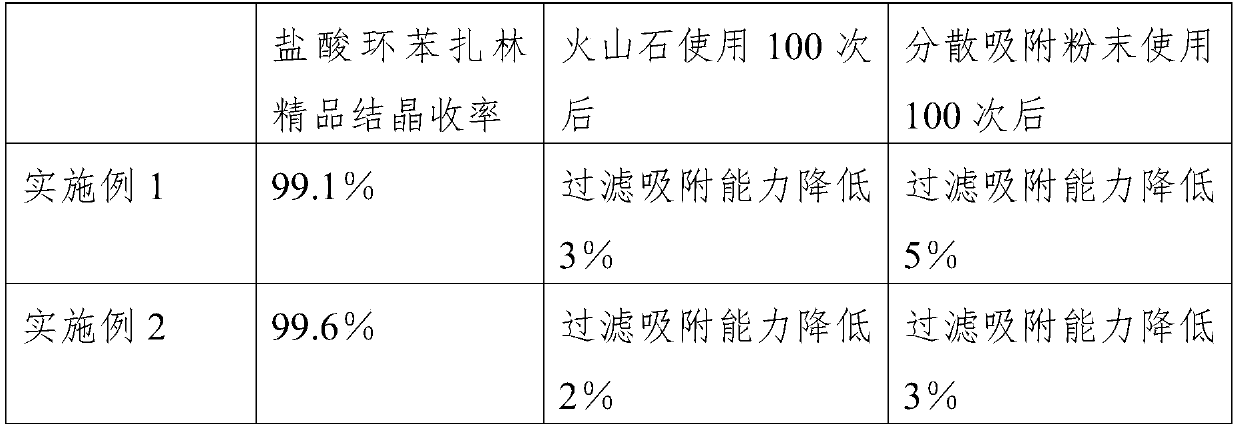
The invention discloses a refining method of cyclobenzaprine hydrochloride, and concretely relates to the field of medicines. The refining method comprises the following preparation steps: S1, in an extraction process in the later period of cyclobenzaprine hydrochloride processing, adding dispersed adsorption powder into a mixed solution of cyclobenzaprine hydrochloride, stirring by adopting a paddle stirrer, and precipitating to obtain a cyclobenzaprine hydrochloride crude product; S2, adding an organic solvent into the cyclobenzaprine hydrochloride crude product, performing recrystallization, dissolving and stirring in a steam pressurizing manner, and keeping constant temperature for later use; and S3, filtering and adsorbing the mixed solution obtained in step S2 through vesuvianite, and filtering to obtain a white refined cyclobenzaprine hydrochloride product. By adding the dispersed adsorption powder, crystals can be dispersed and fluffy, impurity byproducts are not wrapped, crystal discharging is fine and uniform, impurities and redundant metal ions can be adsorbed, and the cyclobenzaprine hydrochloride fine product with high purity and extraction rate is obtained.
Owner:CP PHARMA QINGDAO CO LTD
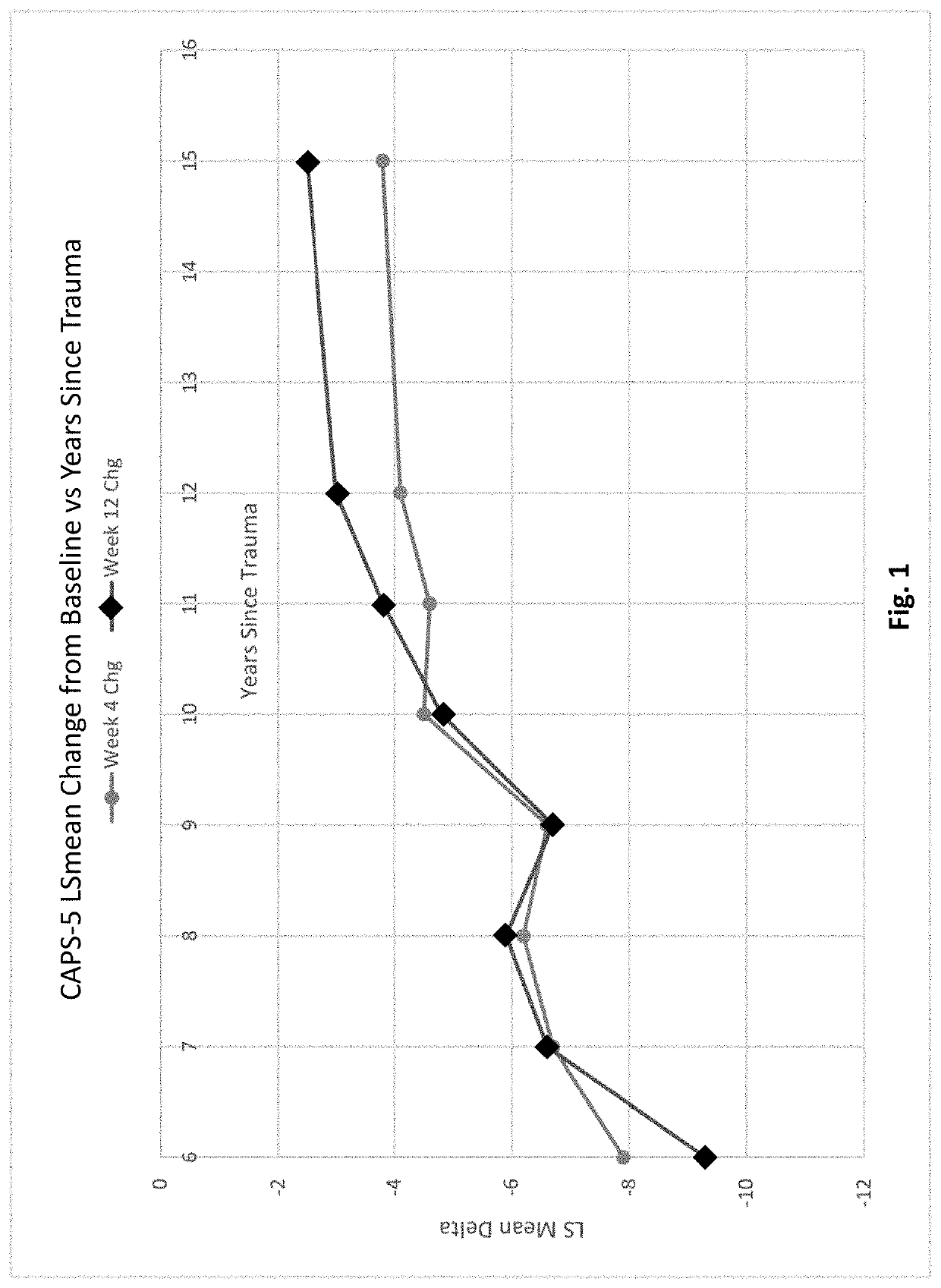
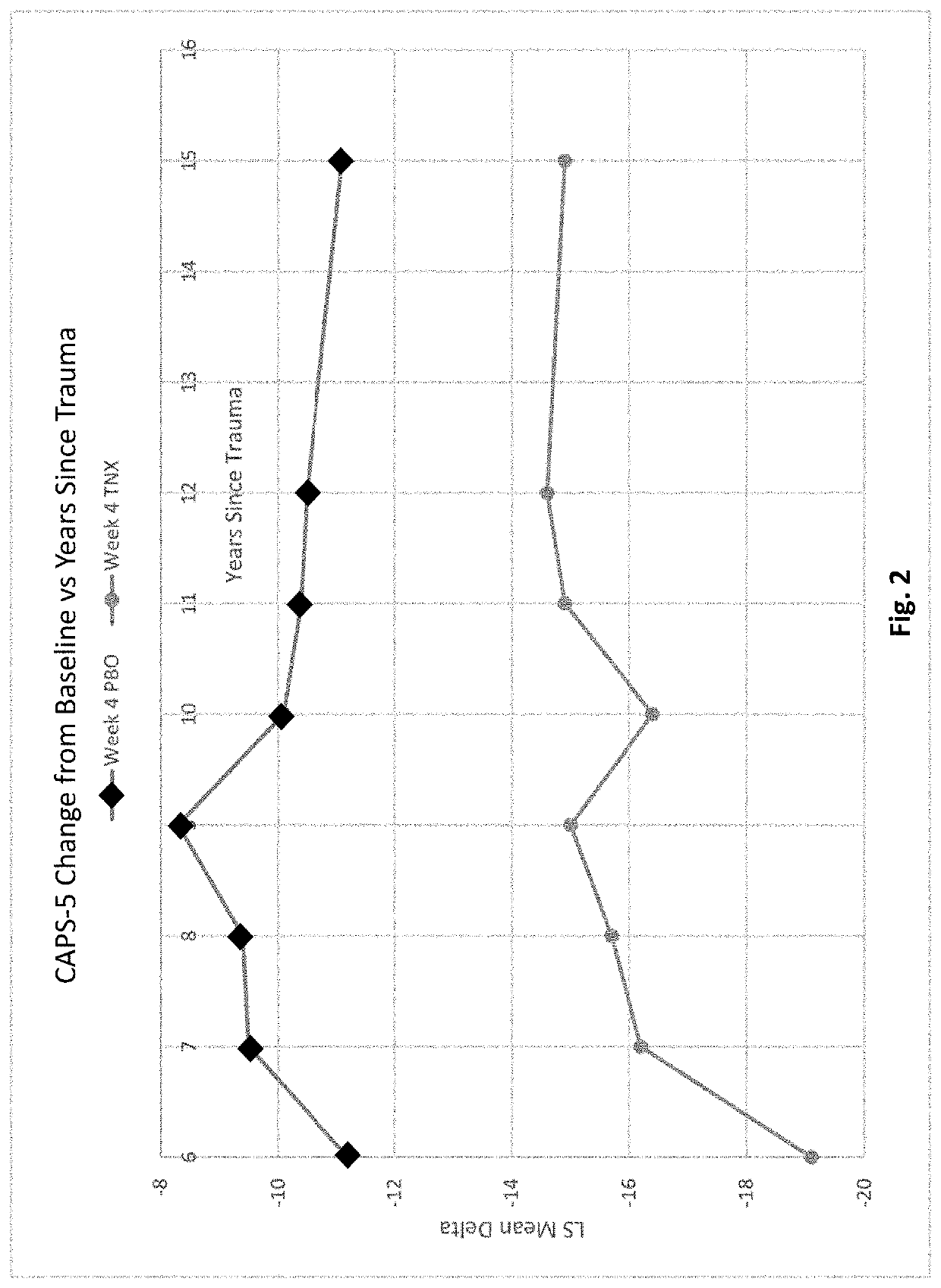
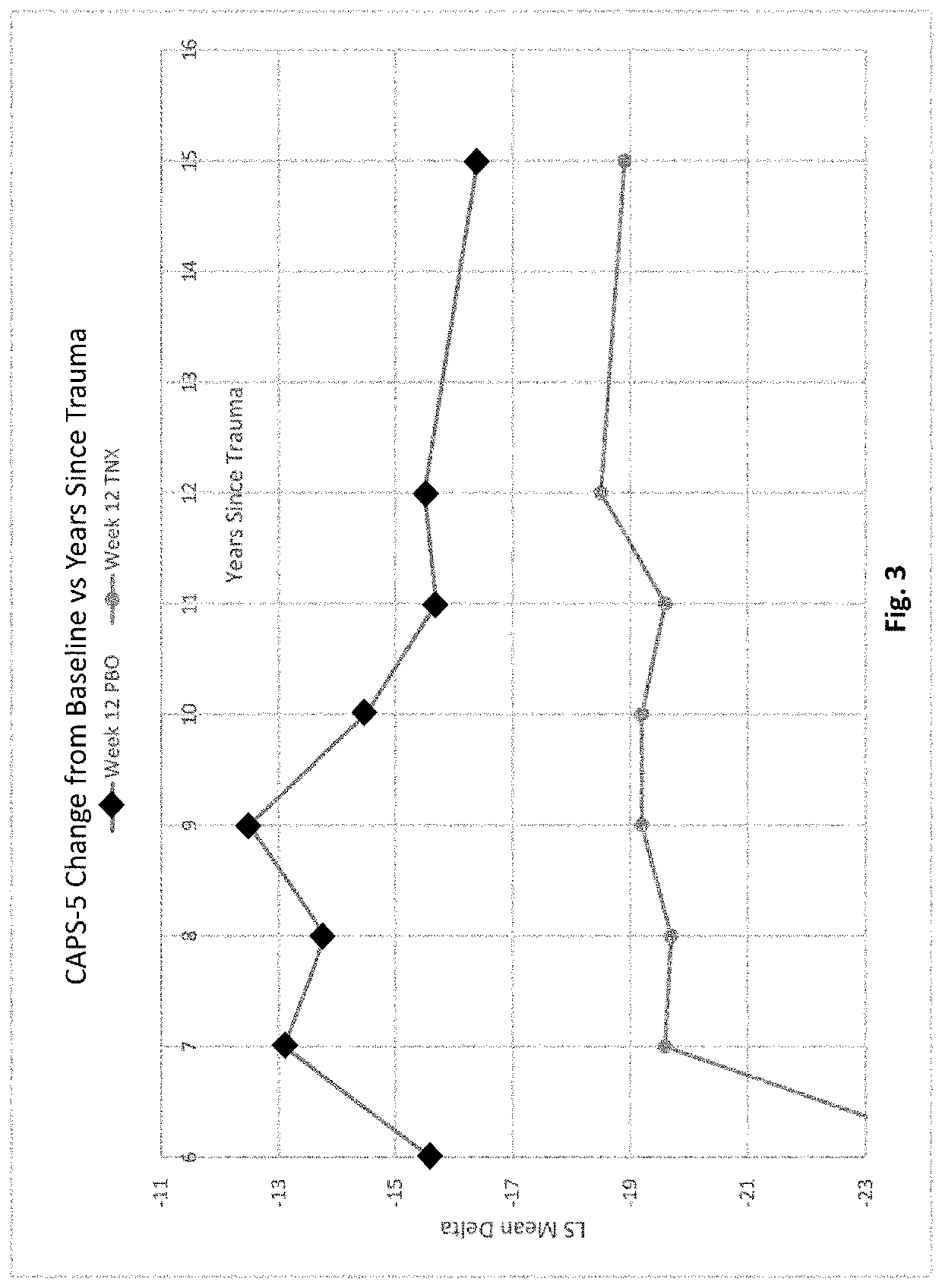
Methods of treating acute stress disorder and posttraumatic stress disorder
PendingCN113194935ARule out suicide riskNervous disorderPharmaceutical delivery mechanismAmitriptylineAcute Stress Disorder
Owner:TONIX PHARMA HLDG LTD
Compound nursing agent for treating fibromyalgia syndrome and preparation method of compound nursing agent
InactiveCN105944083AImprove sleepingInhibit side effectsOrganic active ingredientsPeptide/protein ingredientsTreatment effectS-Adenosyl-l-methionine
The invention discloses a compound nursing agent for treating fibromyalgia syndrome and a preparation method of the compound nursing agent. The compound nursing agent consists of the following ingredients: cyclobenzaprine, amitriptyline, paroxetine, baclofen, propantheline, S-adenosylmethionine, nardosinone, celecoxib, lornoxicam, a basic fibroblast growth factor, methycobal, tryptophan, maltodextrin and casein phosphopeptides. The compound nursing agent disclosed by the invention has functions of diminishing inflammation and relieving pain, soothing liver and relieving depression, dispelling wind and eliminating dampness, clearing and activating channels and collaterals, relieving spasms and improving patients' sleep; and based upon clinical verification, the compound nursing agent is relatively high in cure rate on the fibromyalgia syndrome, good in treatment effect, remarkable in curative effect, low in toxic and side effects and free from clinical adverse reactions, and the compound nursing agent has good values and prospects of clinical popularization and application.
Owner:董桂芬
Cyclobenzaprine hydrochloride sustained-release capsule and preparation method thereof
PendingCN114652700AQuality improvementSmooth releaseOrganic active ingredientsAntipyreticSustained release pelletsAnti-Adhesion Agent
The invention relates to a cyclobenzaprine hydrochloride sustained-release capsule and a preparation method thereof, and belongs to the technical field of sustained-release preparations. The cyclobenzaprine hydrochloride sustained-release capsule is obtained by filling a sustained-release pellet, the sustained-release pellet is composed of a drug-containing pellet core and a coating layer, and the pellet core contains cyclobenzaprine hydrochloride, a filler and a sustained-release framework material; the coating layer contains a sustained-release film-forming material, a plasticizer, a pore-foaming agent and an anti-sticking agent, and the sustained-release pellet consists of a plurality of small unit pellets and is a skeleton type and film control type dual-combined sustained-release capsule. Compared with the prior art, the sustained-release capsule provided by the invention has the advantages of stable quality, more uniform and slow drug release, obviously improved safety, effectiveness and compliance of the drug, simple preparation process, low technical difficulty and suitability for large-scale production.
Owner:LUNAN PHARMA GROUP CORPORATION
Synthesis method of cyclobenzaprine hydrochloride
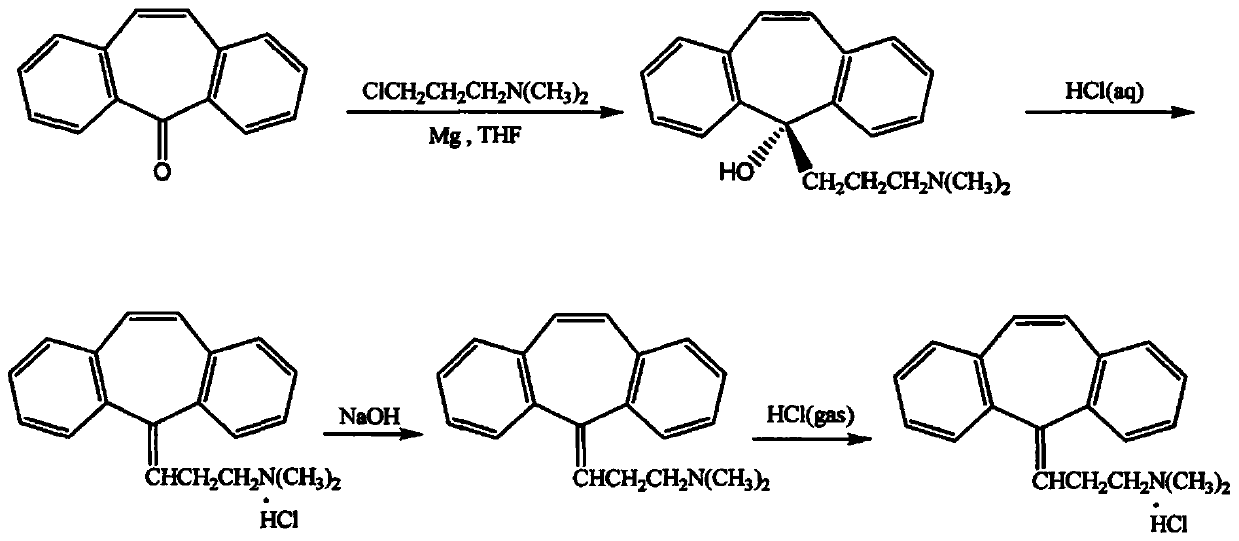
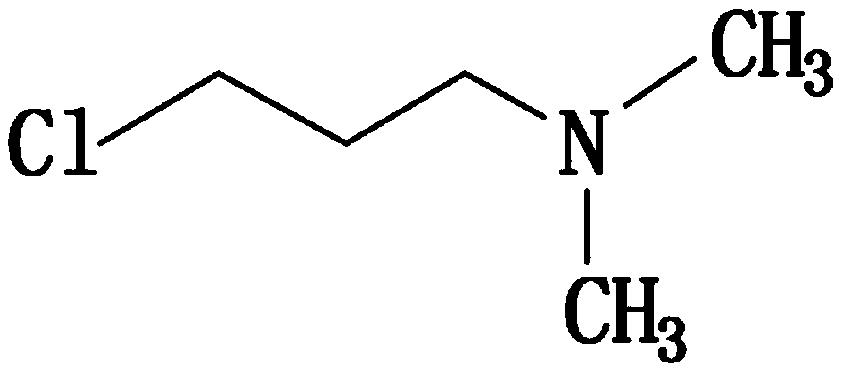
InactiveCN111393305AImprove qualityHigh purityAmino preparation from aminesOrganic compound preparationPropylaminePhysical chemistry
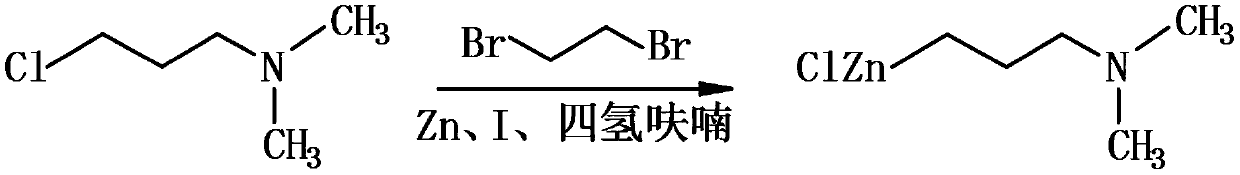
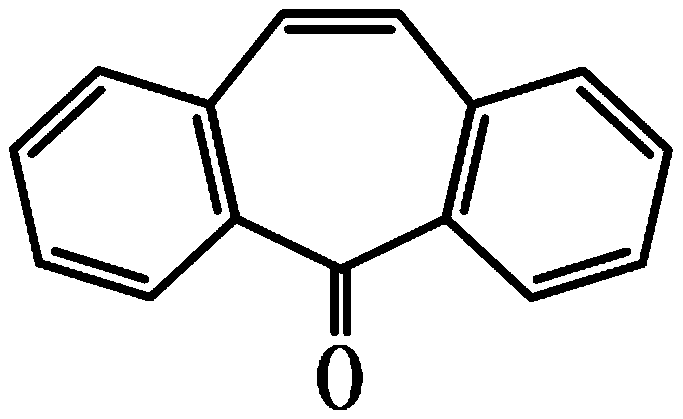
The invention discloses a synthesis method of cyclobenzaprine hydrochloride, and the method comprises: taking 7-10 ml of redistilled tetrahydrofuran, placing the redistilled tetrahydrofuran into a 100ml three-necked bottle, adding 1-2 pieces of iodine particles, 3-5 drops of 1, 2-dibromoethane and 0.7-1.0 g of zinc powder, slowly heating to a temperature of 35 DEG C, and carrying out a constant temperature reaction for 30-40 min. N, N-dimethyl-3-chloro-zinc propylamine is synthesized from N, N-dimethyl-3-chloropropylamine, iodine, 1,2-dibromoethane and zinc powder in tetrahydrofuran, then cyclobenzaprine is synthesized from the N, N-dimethyl-3-chloro-zinc propylamine and 5 H-dibenzo [a, d] cycloheptatriene-5-one, the cyclobenzaprine hydrochloride is synthesized from the cyclobenzaprine, the quality and purity of the cyclobenzaprine hydrochloride are improved by combination of the cyclobenzaprine and a stirring reaction device, a reaction control technology and a washing purification method. The synthetic reaction process is mild, the safety is high, the product yield is high and the purity of the target product is high. The method is simple and feasible, simple, fast, efficient and energy-saving, and is suitable for industrial mass production.
Owner:CP PHARMA QINGDAO CO LTD
Cyclobenzaprine capsule
InactiveCN111494336ASimple preparation processImprove product qualityOrganic active ingredientsMuscular disorderPlasticizerPharmaceutical drug
The invention discloses a cyclobenzaprine enteric capsule and a preparation method thereof. The cyclobenzaprine enteric capsule disclosed by the invention is prepared by loading cyclobenzaprine enteric pellets into a capsule shell, the cyclobenzaprine enteric pellet is composed of a cyclobenzaprine pellet and an enteric coating layer wrapping the cyclobenzaprine pellet, the cyclobenzaprine pelletcomprises cyclobenzaprine and filler, the enteric coating layer comprises an enteric material, a plasticizer and a lubricant, and the preparation method includes: wrapping the cyclobenzaprine pellet with the enteric coating layer and then loading the product into a capsule to obtain the cyclobenzaprine enteric capsule. The cyclobenzaprine enteric capsule provided by the invention is convenient totake, the enteric effect is ideal, the bioavailability of cyclobenzaprine is improved, the preparation process is simple, the obtained product is stable in quality and is suitable for large-scale production.
Owner:CP PHARMA QINGDAO CO LTD +1
Analogs of cyclobenzaprine and amitryptilene
The present invention relates to cyclobenzaprine analogs useful for treatment or prevention of symptoms associated with post-traumatic stress disorder.
Owner:TONIX PHARMA HLDG LTD
Extended release pharmaceutical compositions
ActiveUS9498440B2Reduce morbidityBiocideOrganic active ingredientsSkeletal muscleBuccal administration
Owner:INVENTIA HEALTHCARE LTD
Cyclobenzaprine sustained-release preparation
InactiveCN111494329ASmooth releaseLittle side effectsOrganic active ingredientsMuscular disorderProlonged-release tabletActive agent
The invention discloses a cyclobenzaprine sustained-release tablet and a preparation method thereof. The cyclobenzaprine sustained-release tablet comprises cyclobenzaprine, a sustained-release skeleton matrix, a filler, a surfactant and a lubricant. The medicine provided by the invention can be slowly and uniformly released, achieves the purposes of long acting and curative effect increasing, canalso reduce the dosage and reduce side effects while maintaining the same efficacy, and is simple in preparation process, stable in quality of obtained products and suitable for large-scale production.
Owner:CP PHARMA QINGDAO CO LTD +1
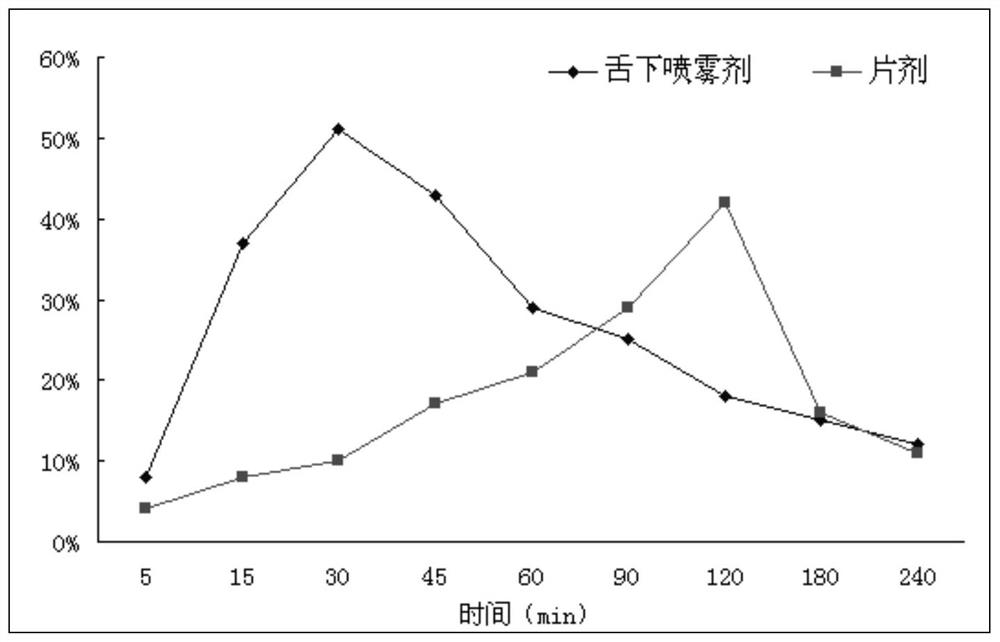
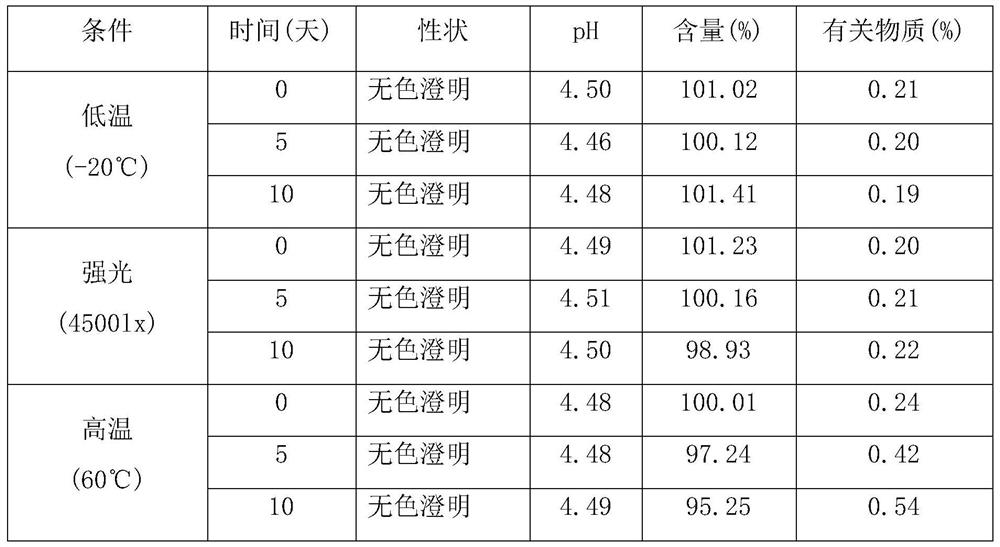
A kind of cyclobenzaprine hydrochloride sublingual spray and preparation method thereof
ActiveCN107496355BImprove applicabilityQuality improvementOrganic active ingredientsAntipyreticPeppermint FlavorDrugs preparations
The invention relates to a cyclobenzaprine hydrochloride sublingual spray and a preparation method thereof, belonging to the field of pharmaceutical preparations. The cyclobenzaprine hydrochloride sublingual spray of the present invention preferably comprises 15 parts of cyclobenzaprine hydrochloride-DM-β-cyclodextrin inclusion compound, 7 parts of vitamins, 3 parts of aspartame, 4 parts of peppermint essence, 0.3 part of acid, appropriate amount of pH regulator, 105 parts of distilled water, the present invention adopts DM-β-cyclodextrin as clathrate material to clathrate cyclobenzaprine hydrochloride to prepare sublingual spray, which is convenient for administration and rapid in action, and The dosage is small, the applicability to patients is strong, the quality is stable, and the effect is remarkable.
Owner:CP PHARMA QINGDAO CO LTD
A kind of cyclobenzaprine hydrochloride sublingual tablet and preparation method thereof
ActiveCN107519142BImprove medication experienceQuality improvementOrganic active ingredientsAntipyreticSucroseCyclobenzaprine hcl
The invention relates to a cyclobenzaprine hydrochloride sublingual tablet and a preparation method thereof, belonging to the field of pharmaceutical preparations. Cyclobenzaprine hydrochloride sublingual tablet of the present invention preferably comprises cyclobenzaprine hydrochloride 25%, diluent 40%, disintegrant 20%, correctives 3%, and the present invention adopts special weight ratio and is 3:2: 5 of sucrose, lactose and mannitol as diluents, and microcrystalline cellulose 10‑20% and carboxymethylcellulose calcium C 10‑20% as disintegrants, the disintegration of sublingual tablets thus prepared can meet the time limit Requirements, the novel form of sublingual tablet is adopted, and it is disintegrated in the oral cavity when taking it, and combined with the preferred mint essence, it can enhance the consumer's medication experience, with stable quality and remarkable effect.
Owner:CP PHARMA QINGDAO CO LTD
Methods of treating acute stress disorder and posttraumatic stress disorder
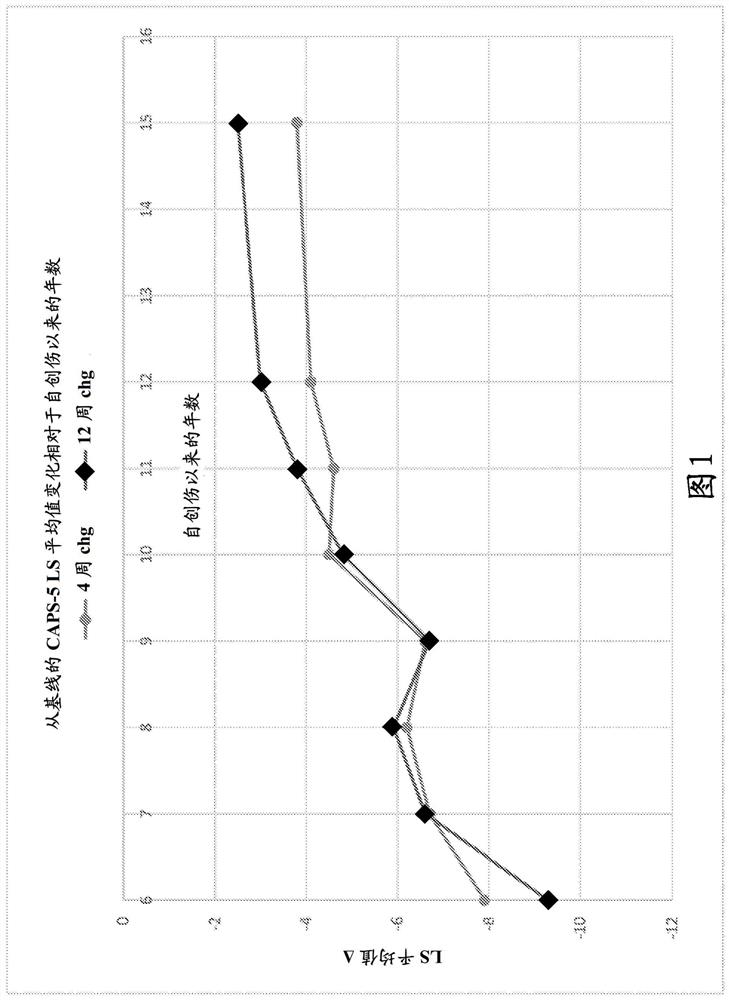
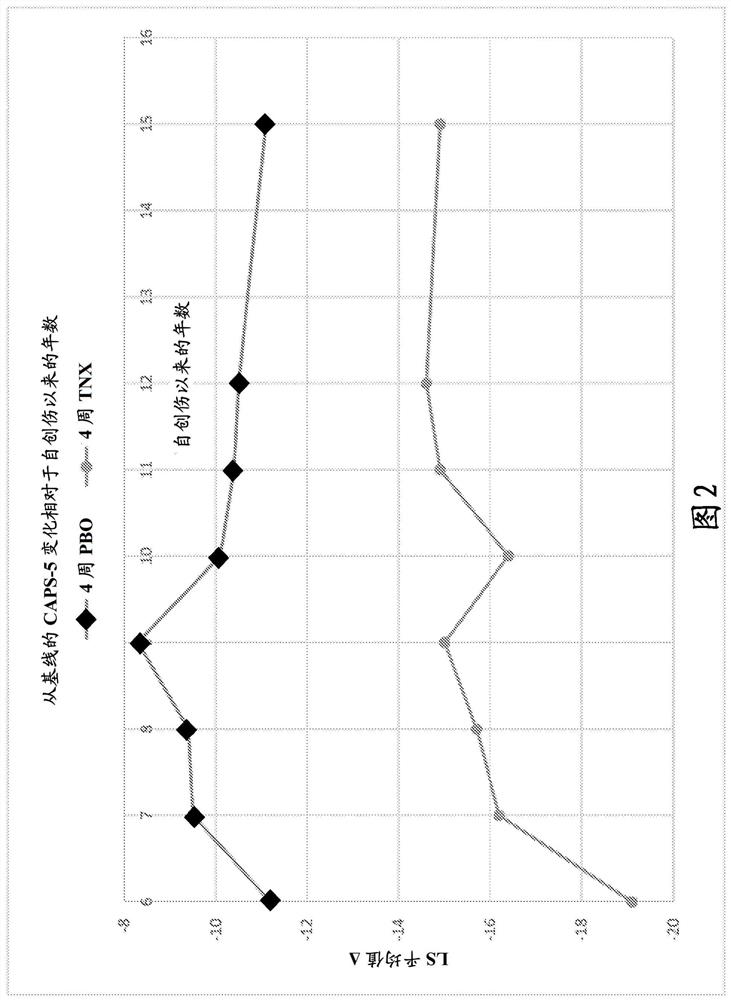
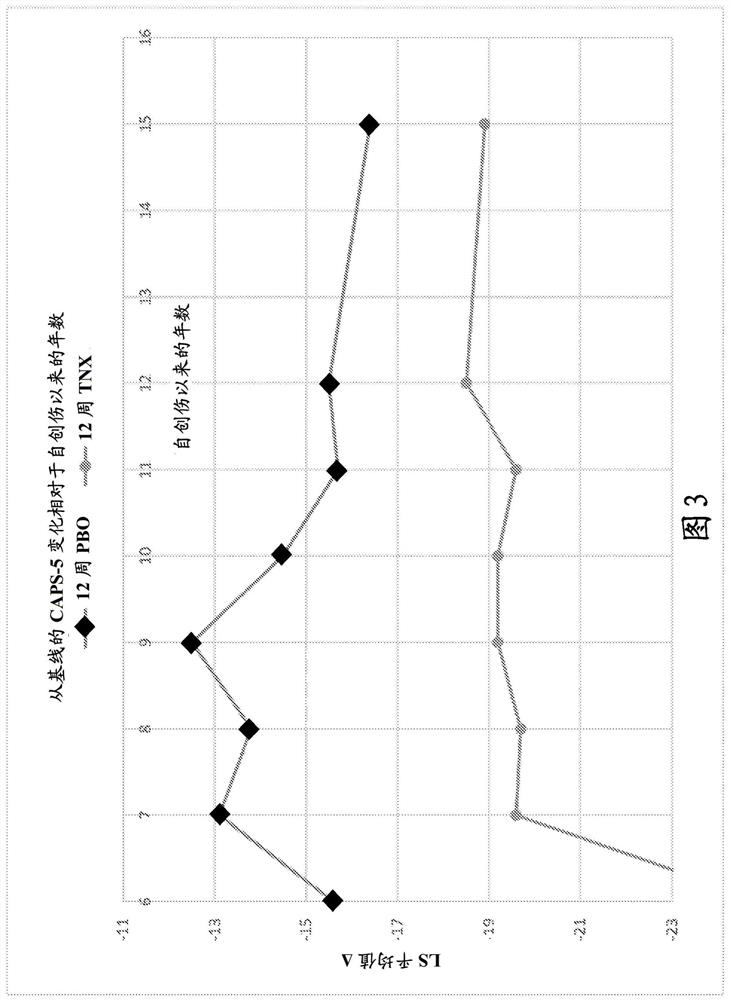
This invention relates to methods of treating posttraumatic stress disorder and acute stress disorder using pharmaceutical compositions comprising cyclobenzaprine, amitriptyline, or pharmaceutically acceptable salts thereof. In particular, it relates to methods of treating posttraumatic stress disorder or one or more symptoms thereof in a subject who has experienced a traumatic event less than or equal to about 9 years prior to the commencement or treatment. It also relates to methods of treating acute stress disorder or one or more symptoms thereof in a subject who has experienced a traumatic event less than or equal to about 1 month prior to the commencement of treatment.
Owner:TONIX PHARMA HLDG LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com