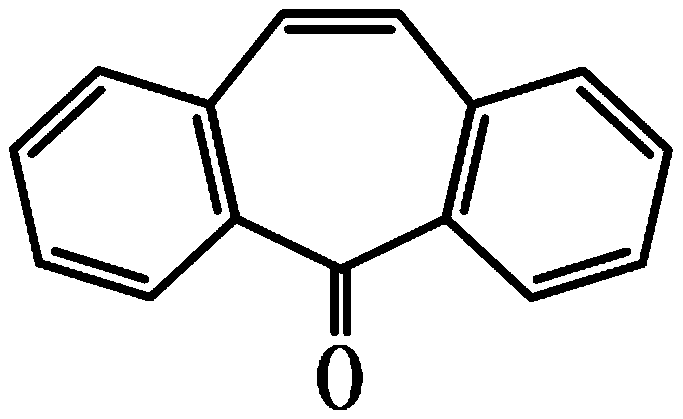
Synthesis method of cyclobenzaprine hydrochloride
A technology of cyclobenzaprine hydrochloride and a synthesis method is applied in chemical instruments and methods, preparation of organic compounds, preparation of aminohydroxy compounds, etc. The effect of mild reaction process, improved quality and purity, and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The invention provides a method for synthesizing cyclobenzaprine hydrochloride, the specific process steps are as follows:
[0036] Step 1: Take 7ml of double-distilled tetrahydrofuran and place it in a 100ml three-neck flask, add 1 iodine tablet, 3 drops of 1,2-dibromoethane and 0.7g of zinc powder, and slowly heat to 35°C for a constant temperature reaction, and the reaction time is 30 minutes;
[0037] Step 2: After the above reaction is complete, add 1.8g of N,N-dimethyl-3-chloropropylamine dropwise, heat the three-necked flask until the solvent inside boils, and condense the steam through the condenser tube and then reflux. The reaction time is set for 3h;
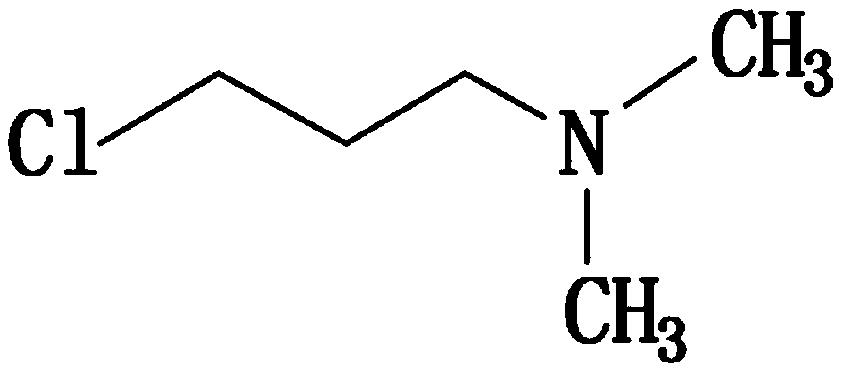
[0038] The general structural formula of the N,N-dimethyl-3-chloropropylamine is:
[0039]
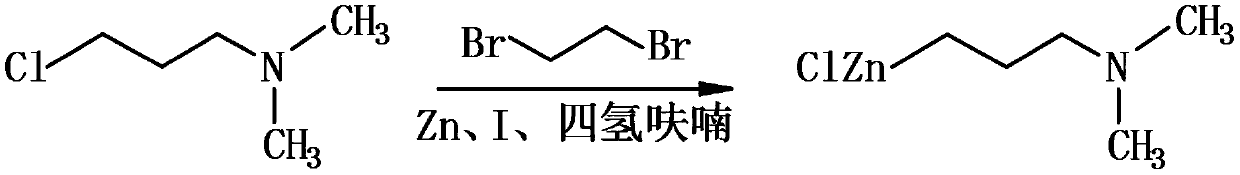
[0040] This reaction synthetic route is as follows:
[0041]
[0042] Wherein, the effective product of the reaction is N,N-dimethyl-3-chlorozincpropylamine;
[0043] Step 3: After the above reaction is complet...
Embodiment 2
[0062] The invention provides a method for synthesizing cyclobenzaprine hydrochloride, the specific process steps are as follows:
[0063] Step 1: Take 8ml of double-distilled tetrahydrofuran and place it in a 100ml three-neck flask, add 1 iodine tablet, 4 drops of 1,2-dibromoethane and 0.9g of zinc powder, and slowly heat to 35°C for a constant temperature reaction. The reaction time is 35 minutes;
[0064] Step 2: After the above reaction is complete, add 1.9 g of N,N-dimethyl-3-chloropropylamine dropwise, heat the three-necked flask until the solvent inside boils, and condense the steam through the condenser tube and then reflux. The reaction time is set 3.2h;
[0065] The general structural formula of the N,N-dimethyl-3-chloropropylamine is:
[0066]
[0067] This reaction synthetic route is as follows:
[0068]
[0069] Wherein, the effective product of the reaction is N,N-dimethyl-3-chlorozincpropylamine;
[0070] Step 3: After the above reaction is complete, th...
Embodiment 3
[0089] The invention provides a method for synthesizing cyclobenzaprine hydrochloride, the specific process steps are as follows:
[0090] Step 1: Take 10ml of double-distilled tetrahydrofuran and place it in a 100ml three-neck flask, add 2 capsules of iodine, 5 drops of 1,2-dibromoethane and 1.0g of zinc powder, and slowly heat to 35°C for a constant temperature reaction. The reaction time is 40min;
[0091] Step 2: After the above reaction is complete, add 2.0 g of N,N-dimethyl-3-chloropropylamine dropwise, heat the three-necked flask until the solvent inside boils, and condense the steam through the condenser tube and then reflux. The reaction time is set 3.5h;
[0092] The general structural formula of the N,N-dimethyl-3-chloropropylamine is:
[0093]
[0094] This reaction synthetic route is as follows:
[0095]
[0096] Wherein, the effective product of the reaction is N,N-dimethyl-3-chlorozincpropylamine;
[0097] Step 3: After the above reaction is complete, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com