Patents
Literature
43 results about "Cycloheptatriene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cycloheptatriene (CHT) is an organic compound with the formula C₇H₈. This colourless liquid has been of recurring theoretical interest in organic chemistry. It is a ligand in organometallic chemistry and as a building block in organic synthesis. Cycloheptatriene is not aromatic, as reflected by the nonplanarity of the methylene bridge (-CH₂-) with respect to the other atoms; however the related tropylium is.
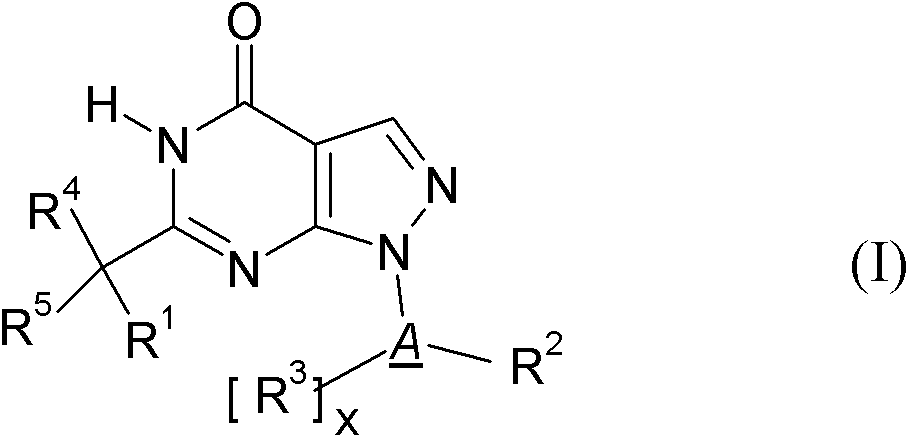
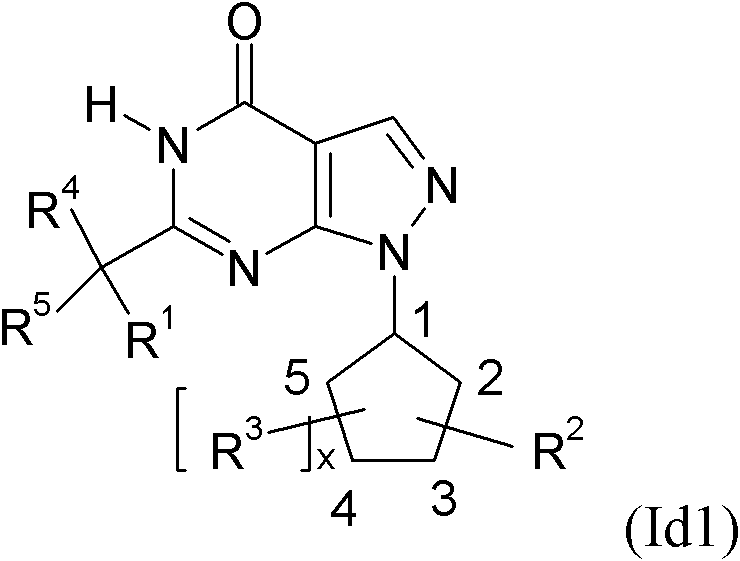
Pyrazolopyrimidines and their use for the treatment of CNS disorders
The invention relates to novel cycloalkyl- or cycloalkenyl-substituted pyrazolopyrimidinones of formula (I), wherein A is selected from the group A1 consisting of a C3-C8-cycloalkyl group or a C4-C8-cycloalkenyl group, whereby the members of C3-C8-cycloalkyl group being selected from the group of cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptanyl and cyclooctanyl; and the members of the C4-C8-cycloalkenyl group, being selected from cyclobutenyl, cyclopentenyl, cyclohexenyl, cycloheptenyl, cyclooctenyl, cyclopentadienyl, cyclohexadienyl, cycloheptadienyl, cyclooctadienyl, cycloheptatrienyl, cyclooctathenyl, cyclooctatetraenyl. The new compounds shall be used for the manufacture of medicaments, in particular medicaments for improving perception, concentration, learning and / or memory in patients in need thereof. Chemically, the compounds are characterised as pyrazolopyrimidinones with a cycloalkyl-moiety directly bound to the 1 position of the pyrazolopyrimidinone and a second substituent in the 6 position which is bound via an optionally substituted methylene-bridge. Further aspects of the present invention refer to a process for the manufacture of the compounds and their use for producing medicaments.
Owner:BOEHRINGER INGELHEIM INT GMBH
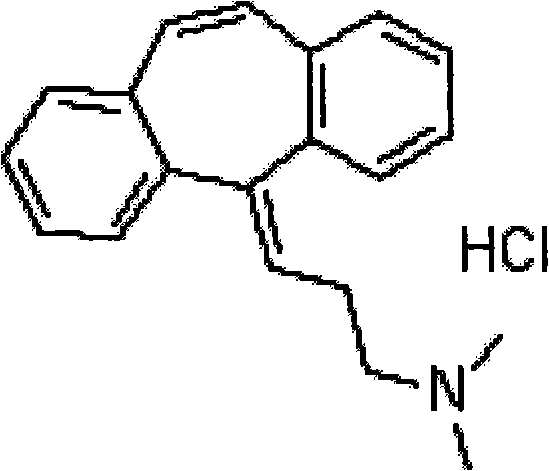
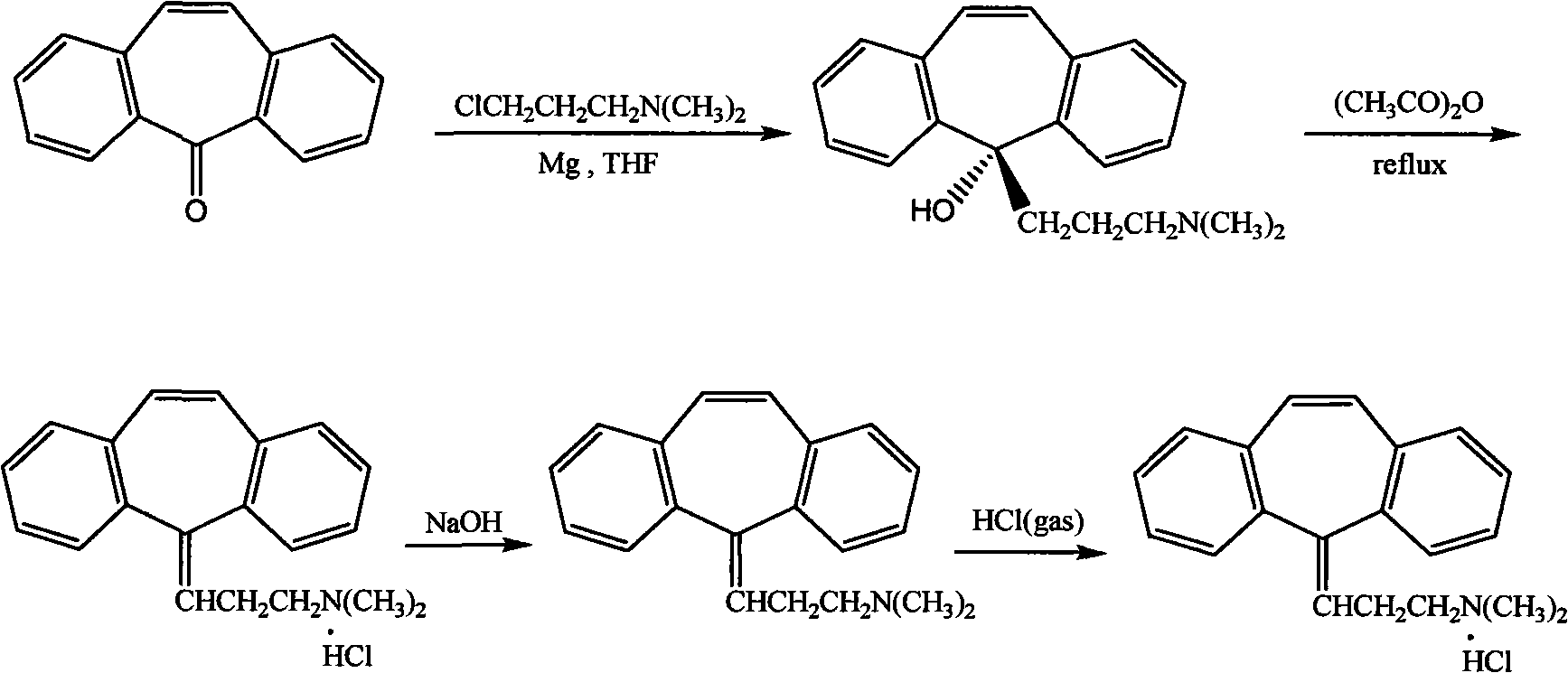
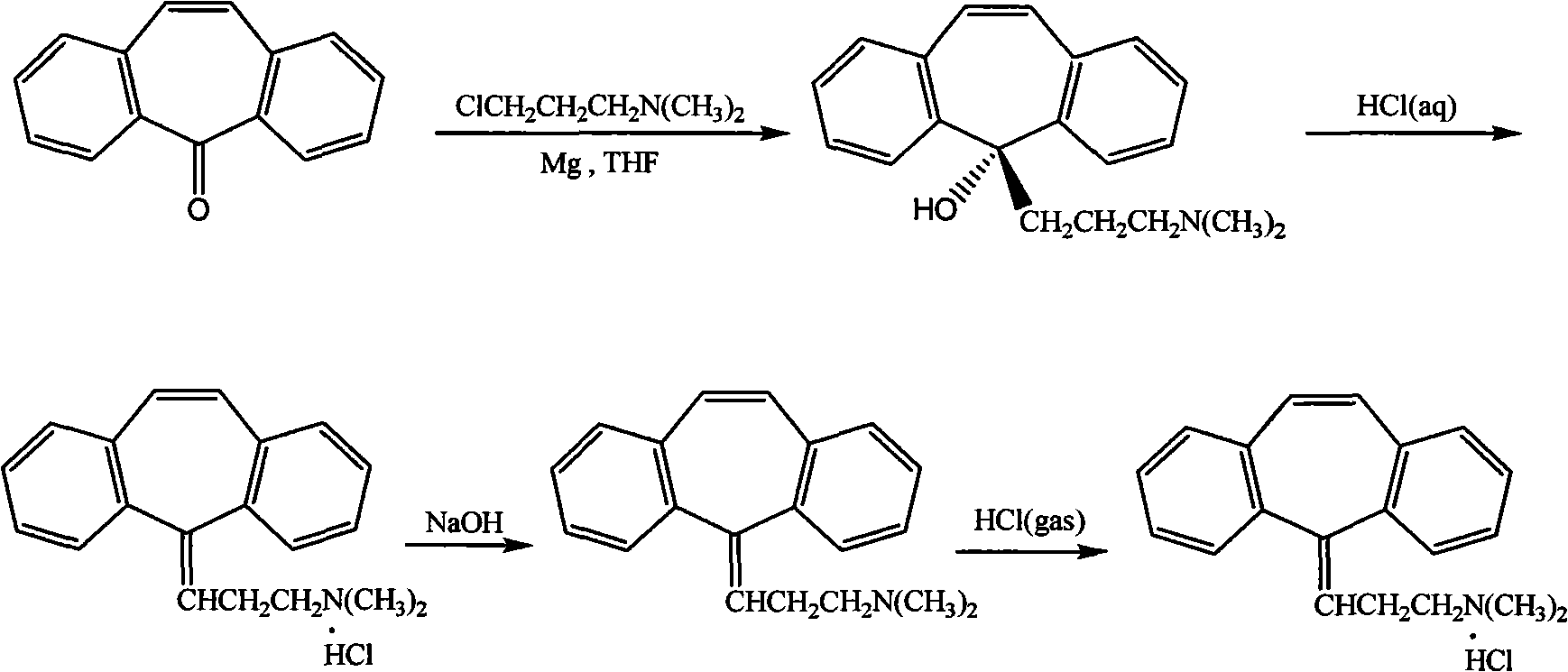
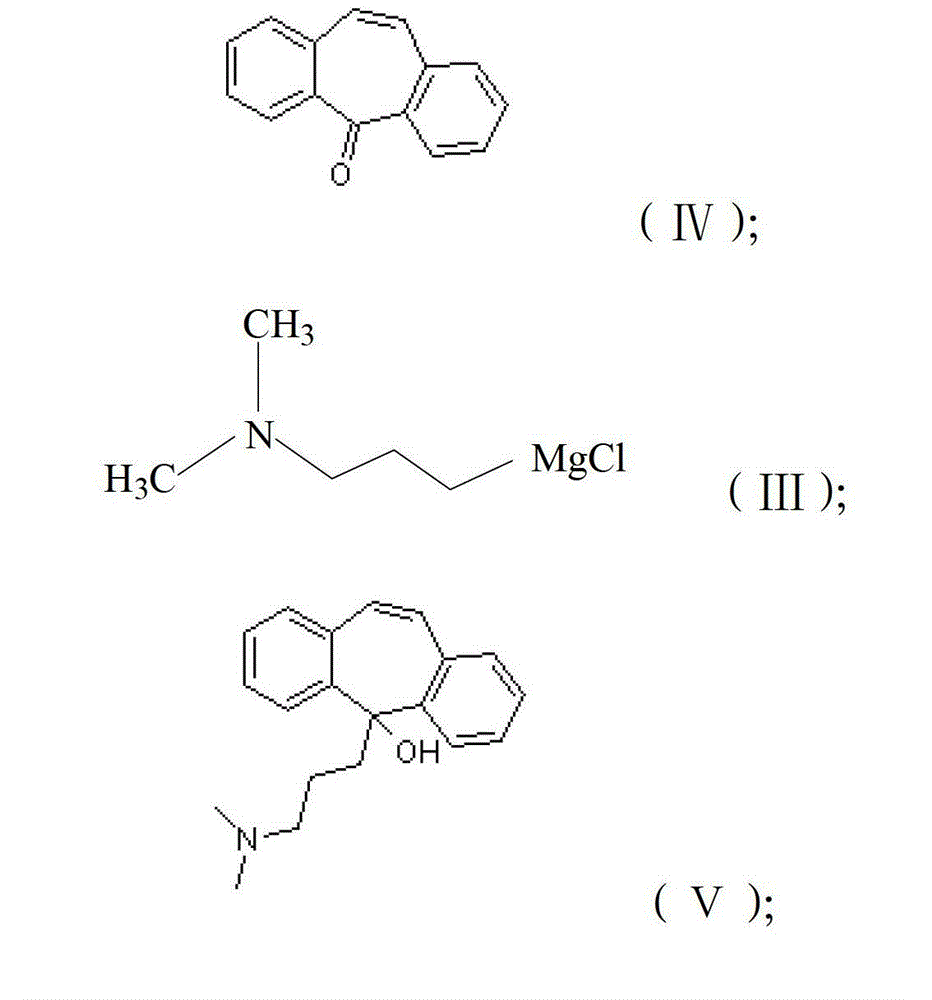
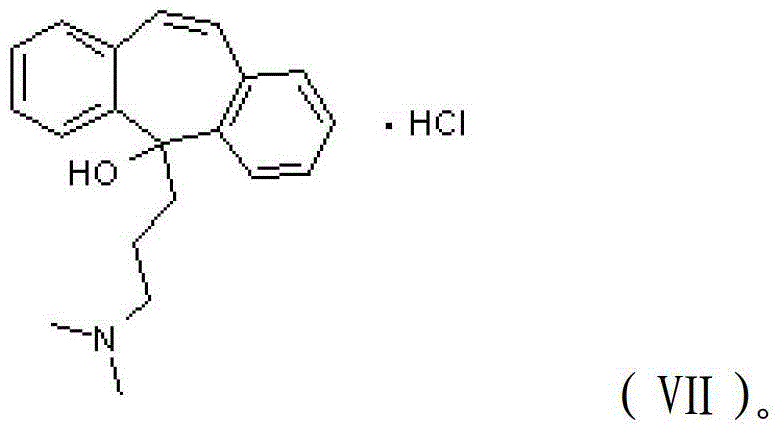
Method for refining cyclobenzaprine hydrochloride
InactiveCN101260046AHigh yieldImprove securityAmino compound purification/separationMuscular disorderOrganic layerEthyl acetate
The invention provides a refinement method for hydrochloric acid cyclobenzaprine, which is safer and more reliable, and can increase the productive efficiency. The refinement method of the hydrochloric acid cyclobenzaprine is as follows: firstly, coarse products of 5- (3- dlmethyl ammonium)- dibenzanthracene (a,d) cycloheptatriene-5-alcohol(cyclobenzaprine intermediate) are dissolved in hydrochloric acids with the concentration of between 1 and 37 percent and heated at a temperature of between 50 and 95 DEG C for 1 to 5 hours, and added in with sodium hydroxide(or other sodium salts) for neutralization so as to make a solution become basic; secondly, an aether / ethyl acetate mixed solution is used for extraction, and the organic layer is filled with dried hydrochloric acid gases for 1 to 5 hours, and coarse products of cyclobenzaprine hydrochloride are separated out; thirdly, coarse products of cyclobenzaprine hydrochloride are recrystallized by absolute isopropanol, and the use level is that each 10g of coarse products is mixed with 30 to 100ml of absolute isopropanol, and the coarse products are filtered, and filter cakes are washed to be nearly white by using acetone, aether or tetrahydrofuran, and coarse products filtered are dried to obtain finished products of cyclobenzaprine hydrochloride, and the content of hydrochloric acid cyclobenzaprine is more than 99.8 percent.
Owner:NANJING HAILING TRADITIONAL CHINESE MEDICINE RES CO LTD +1
Acrylic acid recovery utilizing ethyl acrylate and selected co-solvents
InactiveUS20030146081A1Organic compound preparationSolvent extractionPolymer scienceMethyl palmoxirate
A method of recovering acrylic acid from a mixture comprising acrylic acid, water and acetic acid is disclosed, which includes: (a) extracting acrylic acid from the mixture with a solvent mixture comprising ethyl acrylate as the preponderant component thereof and an organic co-solvent selected from the group consisting of toluene, heptane, 1-heptene, methylcyclohexane, cycloheptane, cycloheptadiene, cycloheptatriene, 2,4-dimethyl-1,3 pentadiene, methylcyclohexene and methylenecyclohexene to form an extracted composition; and (b) azeotropically distilling the extracted composition to recover acrylic acid. Also disclosed is an alternate method of recovering acrylic acid which includes: (a) providing a feed stream containing acrylic acid, water, acetic acid, ethyl acrylate and an organic co-solvent selected from the group consisting of toluene, heptane, 1-heptene, methylcyclohexane, cycloheptane, cycloheptadiene, cycloheptatriene, 2,4-dimethyl-1,3 pentadiene, methylcyclohexene and methylenecyclohexene to a distillation column, wherein the weight ratio of ethyl acrylate to the organic co-solvent is from about 80:20 to about 95:5; and (b) azeotropically distilling said feed stream to provide an acrylic acid residue stream.
Owner:DOW GLOBAL TECH LLC
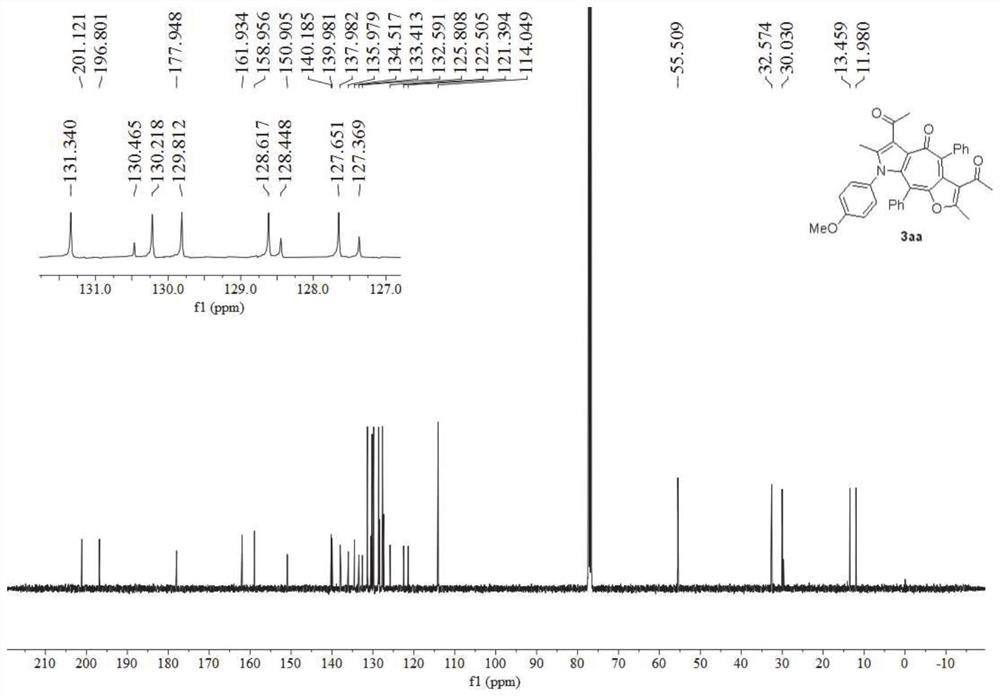
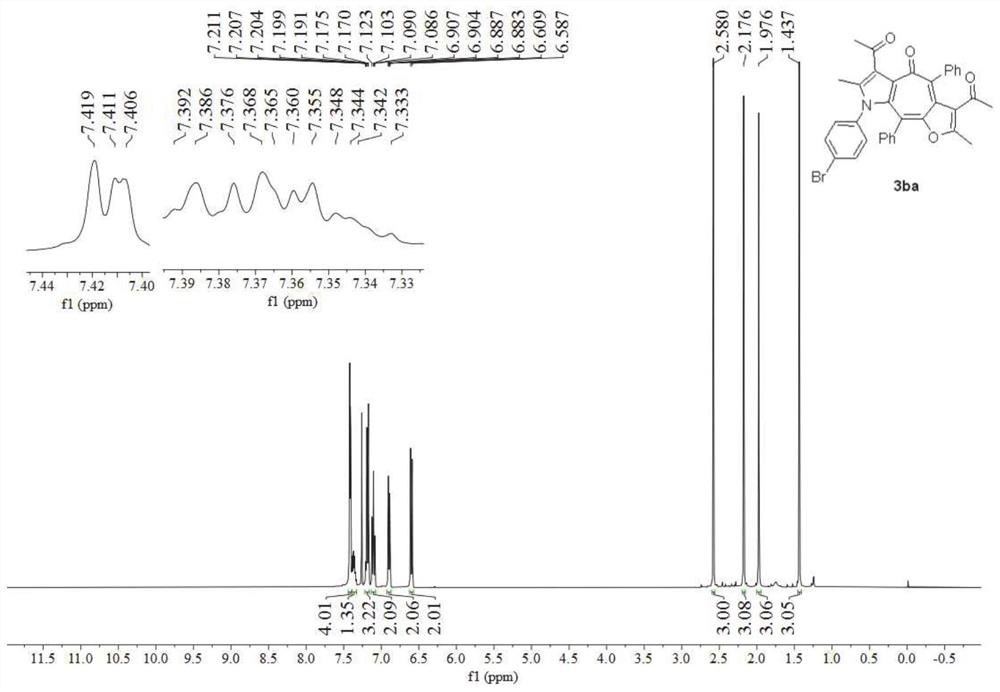
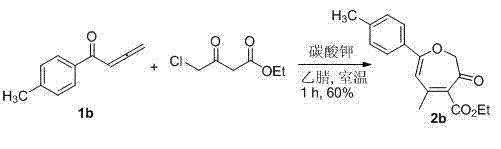
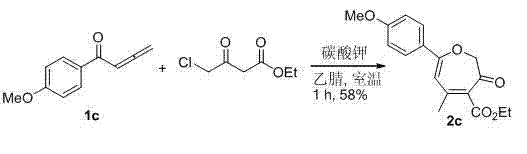
Synthesis method of poly-substituted oxacycloheptatriene-3(2H) ketone compounds
InactiveCN103087033AEasy to separate and purifySimple reaction conditionsOrganic chemistryPtru catalystOrganic solvent
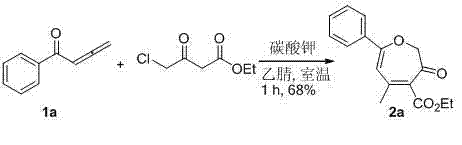
The invention discloses a synthesis method of poly-substituted oxacycloheptatriene-3(2H) ketone compounds. According to the technical scheme, the synthesis method comprises the following steps of: dissolving 4-chloracetyl-ethyl acetoacetate and a 1,2-allenyl ketone compound into an organic solvent; subsequently adding alkali; reacting at room temperature so as to obtain the poly-substituted oxacycloheptatriene-3(2H) ketone compound. The synthesis method is a novel method for synthesizing poly-substituted oxacycloheptatriene-3(2H) ketone compounds, needs no expensive catalysts and reagents, and is simple and easy to prepare starting materials, temperate in reaction condition and simple and convenient to operate.
Owner:HENAN NORMAL UNIV
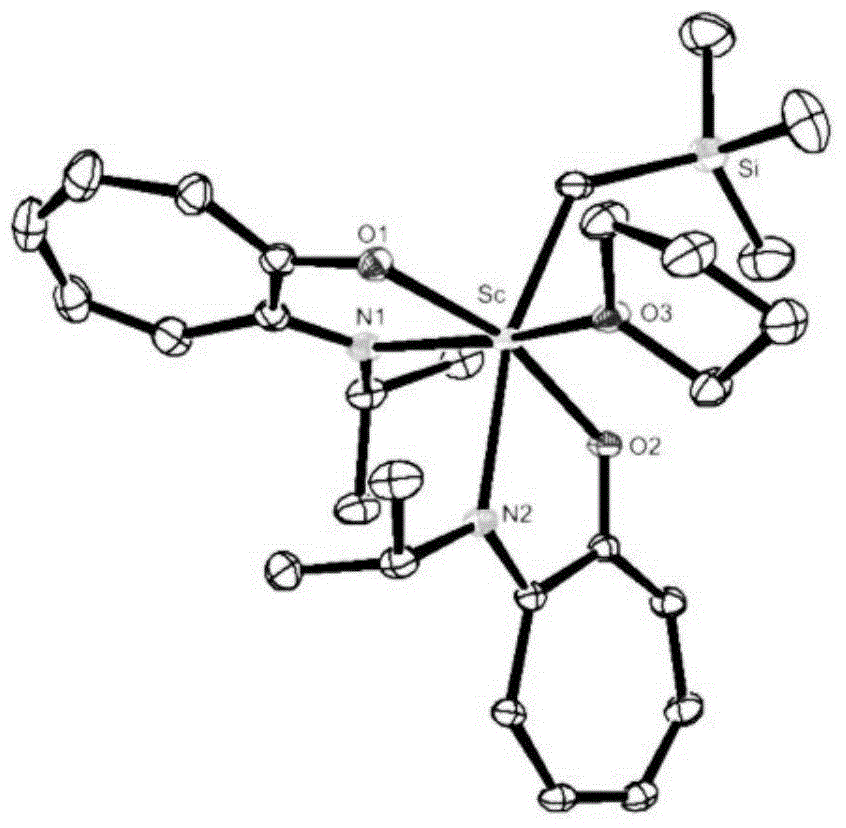
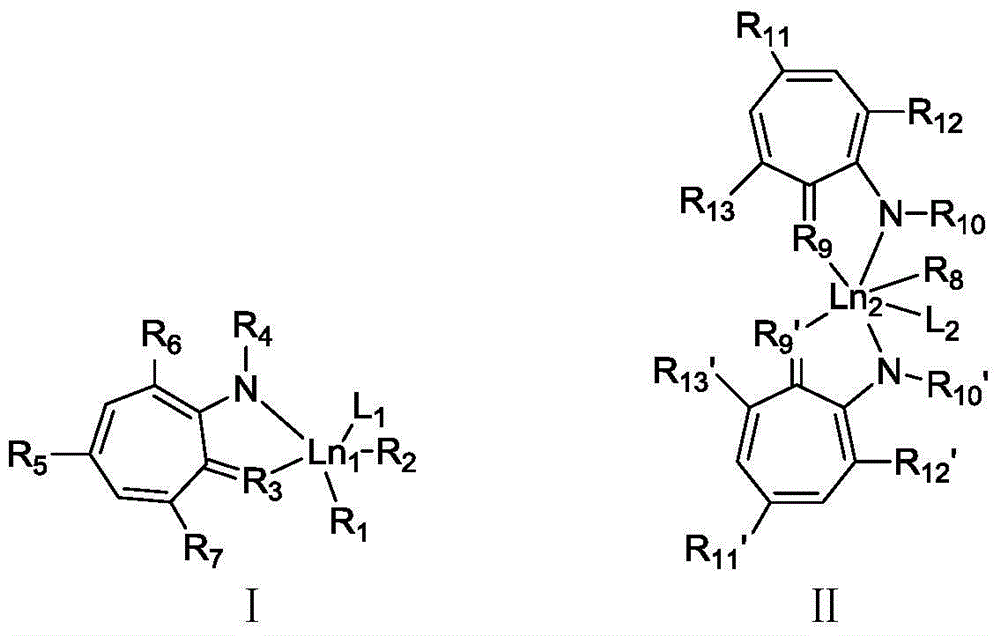
Cycloheptatriene-base rare-earth metal catalyst, and preparation method and application thereof
ActiveCN104592425ARaw materials are cheap and easy to getEasy to retouchGroup 3/13 element organic compoundsRare earthAlkyne
The invention discloses a cycloheptatriene-base rare-earth metal catalyst, and a preparation method and application thereof, belonging to the field of catalysts. The method comprises the following steps: adding tropolone, paratoluensulfonyl chloride and triethylamine into a reactor, reacting in a nitrogen atmosphere for some time, adding amino substitute, reacting over night, recrystallizing to obtain a pure product, reacting with an Et3OBF4 solution for several hours, dropwisely adding the amino substitute, stirring over night, purifying by column chromatography to obtain a cycloheptatriene-base ligand; and dropwisely adding the ligand into an LnR3-dissolved toluene solution, stirring to react at room temperature for some time, filtering, concentrating, and recrystallizing to obtain the cycloheptatriene-base rare-earth metal catalyst. The catalyst has the advantage of accessible raw materials, and is applicable to polymerization reaction of multiple monomers, including homopolymerization and copolymerization of olefins, alkynes and polar monomers or copolymerization with CO2. The preparation method is simple, economical and environment-friendly, has wide application range, and is suitable for industrial production.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Acrylic acid recovery utilizing ethyl acrylate and selected co-solvents
A method of recovering acrylic acid from a mixture comprising acrylic acid, water and acetic acid is disclosed, which includes: (a) extracting acrylic acid from the mixture with a solvent mixture comprising ethyl acrylate as,the preponderant component thereof and an organic co-solvent selected from the group consisting of toluene, heptane, 1-heptene, methylcyclohexane, cycloheptane, cycloheptadiene, cycloheptatriene, 2,4-dimethyl-1,3 pentadiene, methylcyclohexene and methylenecyclohexene to form an extracted composition; and (b) azeotropically distilling the extracted composition to recover acrylic acid. Also disclosed is an alternate method of recovering acrylic acid which includes: (a) providing a feed stream containing acrylic acid, water, acetic acid, ethyl acrylate and an organic co-solvent selected from the group consisting of toluene, heptane, 1-heptene, methylcyclohexane, cycloheptane, cycloheptadiene, cycloheptatriene, 2,4-dimethyl-1,3 pentadiene, methylcyclohexene and methylenecyclohexene to a distillation column, wherein the weight ratio of ethyl acrylate to the organic co-solvent is from about 80:20 to about 95:5; and (b) azeotropically distilling said feed stream to provide an acrylic acid residue stream. A further embodiment of this invention involves directing the recovered acrylic acid stream to a distillation tower wherein a vapor or liquid side stream is obtained having a purity level of acrylic acid of at least 99%. This material can be subsequently further purified to obtain glacial acrylic acid having a purity of at least 99.8%.
Owner:DOW GLOBAL TECH LLC +1
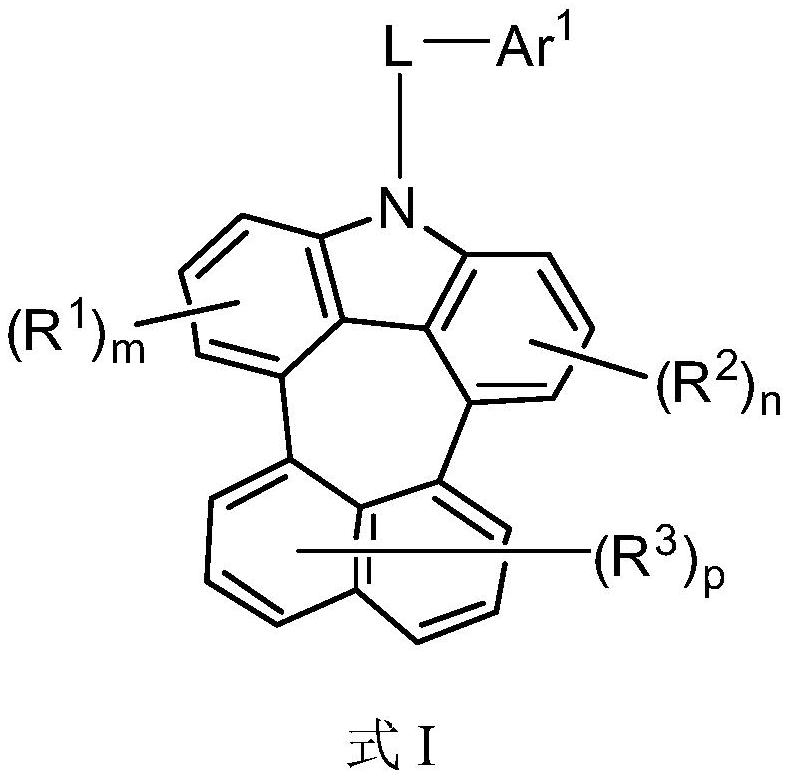
Compound and application thereof
PendingCN112979535AGood planarityLower transmission barriersOrganic chemistrySolid-state devicesCarbazoleLow voltage
The invention relates to a compound and application thereof. The compound has a structure as shown in a formula I. By introducing a carbazole structure of cycloheptatriene, the planarity of molecules is improved, and the transmission barrier of current carriers is reduced, so that an organic electroluminescent device comprising the compound has the advantages of low voltage and high efficiency.
Owner:BEIJING ETERNAL MATERIAL TECH
Anion environment-friendly adhesive based on acrylic resin and preparation method of anion environment-friendly adhesive
ActiveCN108276935ALower the volumeLow costNon-macromolecular adhesive additivesAcid polymer adhesivesEnvironmental resistanceAdhesive
The invention discloses an anion environment-friendly adhesive based on acrylic resin and a preparation method of the anion environment-friendly adhesive. The anion environment-friendly adhesive is prepared by pre-polymerizing the following components in parts by weight until the viscosity reaches 1000 to 30000mpa.s, then rapidly cooling and stopping polymerizing. The anion environment-friendly adhesive is prepared from the following components: 30 to 300 parts of acrylic monomer or methacrylic monomer, 0 to 300 parts of other free radical polymeric monomers without carboxylic acid groups, 1 to 60 parts of negative oxygen ion powder, 0.1 to 5 parts of an initiator for free radical polymerization, 0.1 to 5 parts of cycloheptatriene phenolic acetone copper salt and 0.1 to 10 parts of a dispersing agent. The negative oxygen ion powder is coated with carboxyl on the acrylic resin monomer to form a micelle with a core-shell structure; the micelle is heated to be pre-polymerized to form theenvironment-friendly adhesive with proper viscosity; the negative oxygen ion powder positioned on the core of the micelle endows the environment-friendly adhesive with a negative oxygen ion release function, so that the inhibition of formaldehyde release can be realized. The cycloheptatriene phenolic acetone copper salt is an efficient broad-spectrum antibacterial agent and can be used for inhibiting the growth of most harmful microorganisms in the living environment and realizing the functions of preventing mildew and resisting viruses.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
Cockroach pesticide
InactiveCN105494346AMass killing effectEfficient killingBiocideAnimal repellantsBenzoic acidAminopropionitrile
The invention relates to a pesticide, and specifically relates to a cockroach pesticide. The cockroach pesticide is prepared from the following raw materials in parts by weight: 25 to 30 parts of 3-[(2-chlorophenyl)amino]propionitrile, 15 to 18 parts of 1-methyl-4-(5H-dibenzo[a,d]cycloheptatriene-5-ylidene)piperidine hydrochloride, 25 to 28 parts of tranylcypromine hydrochloride, 30 to 32 parts of 4-acetamido-2-ethyoxylmethyl benzoate, and 12 to 18 parts of 1,4-butanediol monomethyl ether. The chemical components cooperate with each other to generate a great killing performance and thus the cockroaches can be killed effectively.
Owner:张媛媛
Preparation method of hydrochloric acid cyclobenzaprine
Owner:佛山市隆信医药科技有限公司
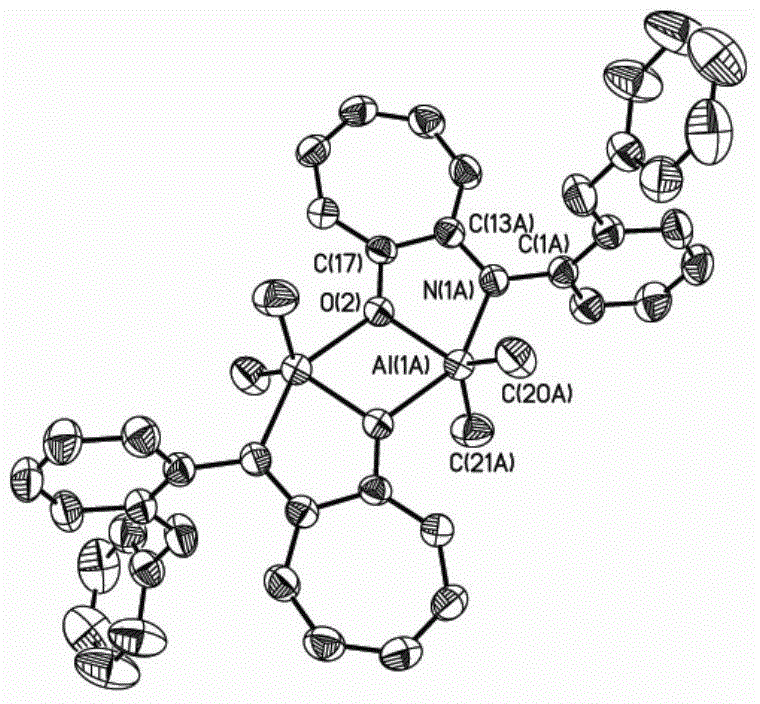
Cycloheptatriene-structure-containing aluminium compound catalysts, synthesis thereof and uses of the catalysts
InactiveCN105732406AOrganic compound preparationGroup 3/13 element organic compoundsLactideHigh activity
The invention relates to an aluminum compound catalyst containing a cycloheptatriene structure, its synthesis and application, and specifically discloses a ring-opening polymerization of lactide catalyzed by an aluminum compound containing a cycloheptatriene structure. Such catalysts can efficiently catalyze the ring-opening polymerization of lactide. The high activity of such catalysts may be due to the formation of smaller chelate rings with aluminum and greater ring tension. The activity of the catalyst containing the side arm effect is significantly higher than that of the catalyst without the side arm effect. When the monomer ratio is 100:1 and the temperature is 80°C, the lactide can be almost completely polymerized. At the same time, the side arm effect also affects the tacticity of PLA.
Owner:UNIV OF SCI & TECH OF CHINA
Water treatment chemical for removing para-nitroaniline in water
InactiveCN104891625AStrong complexing abilityFast precipitationWater contaminantsWater/sewage treatment by flocculation/precipitationPara-nitroanilineP-Nitroaniline
The invention relates to water treatment chemical for removing para-nitroaniline in water. The water treatment chemical is made from 3, 7-dimethyl-6-octenal, 4-formylphenylBeta-D-allopyranoside, aluminum sulfate, trans, trans-2, 4-nonadienal, 2-hydroxyl-4-isopropyl-2, 4, 6-cycloheptatriene-1-one, apha-aminoisocaproic acid, potassium alginate, picrasinol B, (S)-2-(fmoc-amino)caproic acid, acutumidine, 1-pentadecanol, 3, 7, 11-trimethyl-1, 6, 10-dodecatriene-3-ol, amentoflavone, and 2-ethyl-2, 5-dimethylhexanoic acid. The water treatment chemical has the advantages that the chemical has high ability to complex with the para-nitroaniline, complex precipitant forms fast, the removal rate of the para-nitroaniline is up to 99%, and the chemical is low in toxicity, low in use amount, free of harm to water and low in treatment cost.
Owner:金双蕾
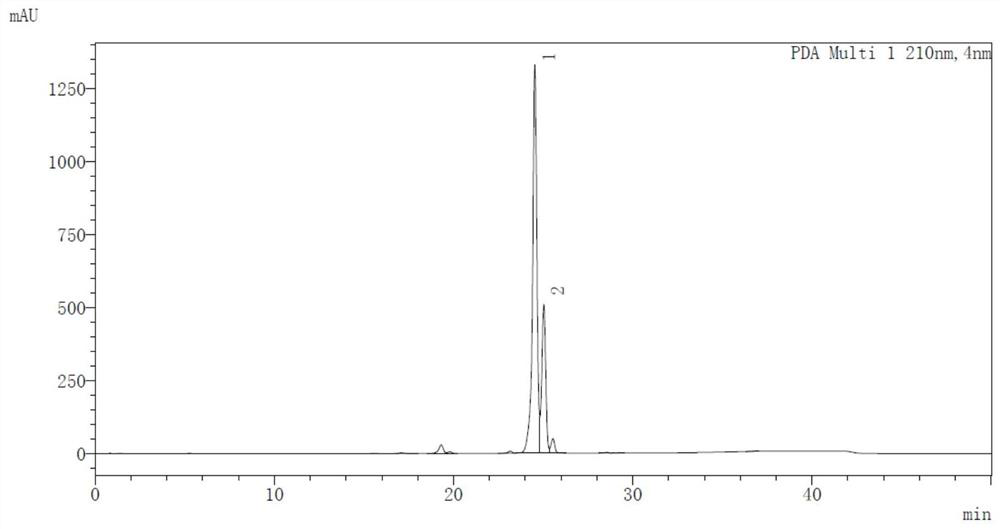
10-methoxy-4H-benzo[4,5]cycloheptatriene[1,2-b]thiazol-4-one preparation method
ActiveCN104817532AEasy to operateEasy post-processingOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsRefluxBenzene
The present invention discloses a 10-methoxy-4H-benzo[4,5]cycloheptatriene[1,2-b]thiazol-4-one preparation method, which comprises: mixing a raw material 9,10-dihydro-9,10-dibromo-4H-benzo[4,5]cycloheptatriene[1,2-b]thiazol-4-one and absolute methanol, carrying out stirring reflux for 2-6 h, adding a solid alkali catalyst to the reaction solution, continuously carrying out the reflux reaction for 2-6 h, carrying out separation purification on the reaction solution after completing the reaction, and recovering the solid alkali catalyst so as to obtain the product 10-methoxy-4H-benzo[4,5]cycloheptatriene[1,2-b]thiazol-4-one. According to the present invention, the solid alkali catalysis is used, the technology is easy to operate, the equipment is not corroded, the three-waste is less, the post-treatment is easy, the solid alkali can be reused, and the preparation method is the economical and practical green environmental protection technology.
Owner:湖州优研知识产权服务有限公司
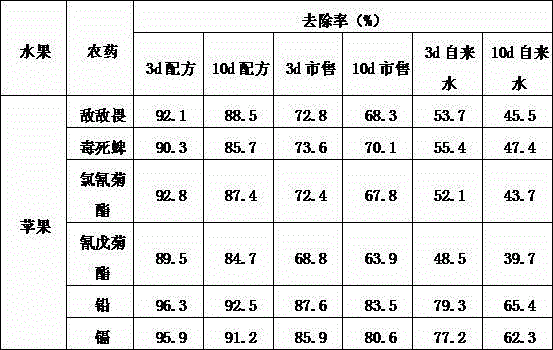
Apple detergent
InactiveCN104450267ASolve the defect of insufficient cleaningImprove cleaning efficiencyNon-ionic surface-active compoundsDetergent compounding agentsGlutaric acidDimethyl acetal
The invention relates to a detergent, particularly an apple detergent. The apple detergent is prepared from the following raw materials in parts by weight: (6-aminohexyl)carbamic acid, 3,7-dimethyl-3-octanol, 3,7-dimethyl-1,6-octadiene-3-ol acetate, 2-hydroxy-4-isopropyl-2,4,6-cycloheptatriene-1-one, 1,3-butanediol dimethylacrylate, 1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride, glycollic aldehyde dimethyl acetal and disodium 2-oxo-1,5-glutarate dihydrate. The apple detergent is free of toxic substances, and can not hurt the human body. The detergent has favorable cleaning effect, can effectively remove organic phosphorus pesticides, pyrethrin pesticides, heavy metals (lead and cadmium) and other harmful substances attached to the apple surface, and ensures the edible safety of the cleaned apple.
Owner:徐玉文
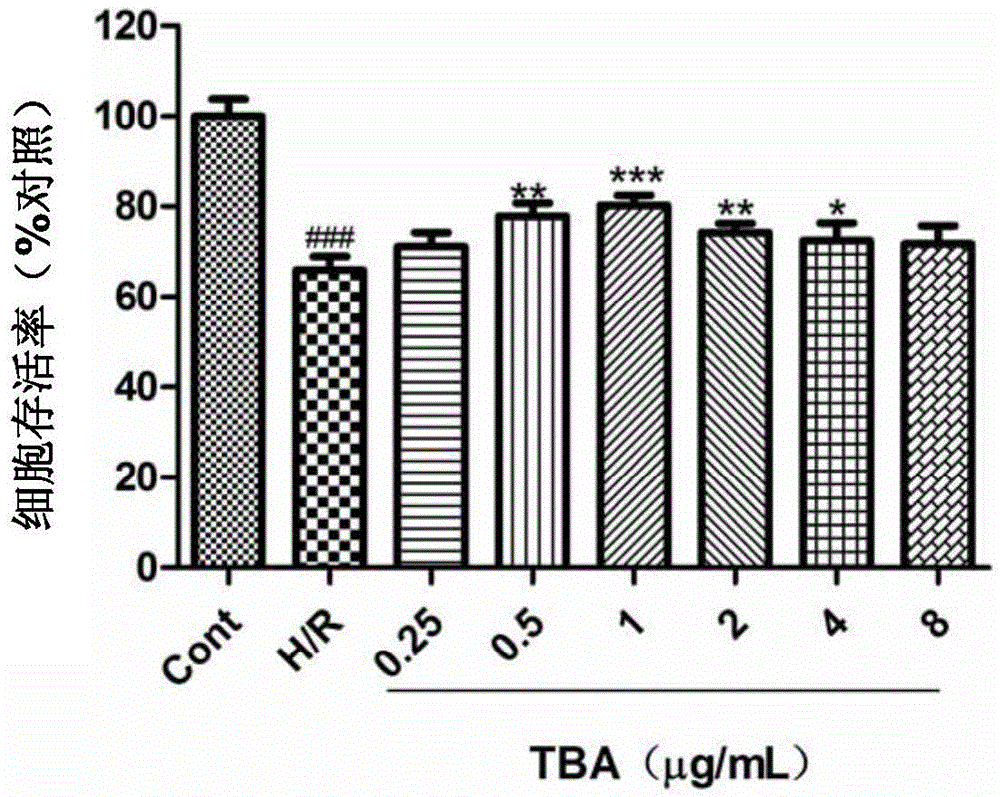
Application of 1,3,4,5-tetrahydro-2-benzo-cycloheptatriene compound to in vitro screening or drug preparation
ActiveCN105534971AAvoid damageProtection from damageOrganic active ingredientsOrganic chemistryDrugs preparationsStructural formula
The invention provides application of a 1,3,4,5-tetrahydro-2-benzo-cycloheptatriene compound to in vitro screening or drug preparation. The 1,3,4,5-tetrahydro-2-benzo-cycloheptatriene compound is a compound shown by the structural formula (I) or pharmaceutically-acceptable salt of the compound shown by the structural formula (I), wherein R1 (please see the formula in the description), R2 and R3 are independently selected from -OH, OCH3, H or C1-C3 alkyl groups, and R4 is C1-C3 alkyl groups, C1-C3 carboxyl groups, C1-C3 carbonyl groups, C1-C3 aldehyde groups or C1-C3 ester groups. The compound can reduce myocardial cell injuries caused by hpoxia and reoxygenation, has a remarkable effect on protecting myocardial cell injuries and is suitable for being applied to in vitro screening or preparing drugs for treating and / or preventing diseases relevant to myocardial cell injuries.
Owner:INST OF MEDICINAL PLANT DEV CHINESE ACADEMY OF MEDICAL SCI
Analytical method for determining cycloheptatriene peptide anthelmintic intermediates
PendingCN113686978AImprove the efficiency of process optimizationEfficient separationComponent separationAnthelmintic drugSilica gel
The invention relates to a method for determining purity and impurities of an emodepside intermediate 1 through high performance liquid chromatography, and relates to the technical field of medicines. According to the technical scheme, a sample is prepared into a test solution by a proper method, methanol-tetrahydrofuran-water is taken as a mobile phase, a main component, namely, an emodepside intermediate 1 and related impurities are separated on a pentafluorophenyl bonded silica gel column, and an ultraviolet detector is used for detection. The method can be used for effectively separating the isomer of the emodepside intermediate 1, and the efficiency of optimizing the emodepside process is improved.
Owner:CHONGQING QIANTAI BIOLOGICAL MEDICINE
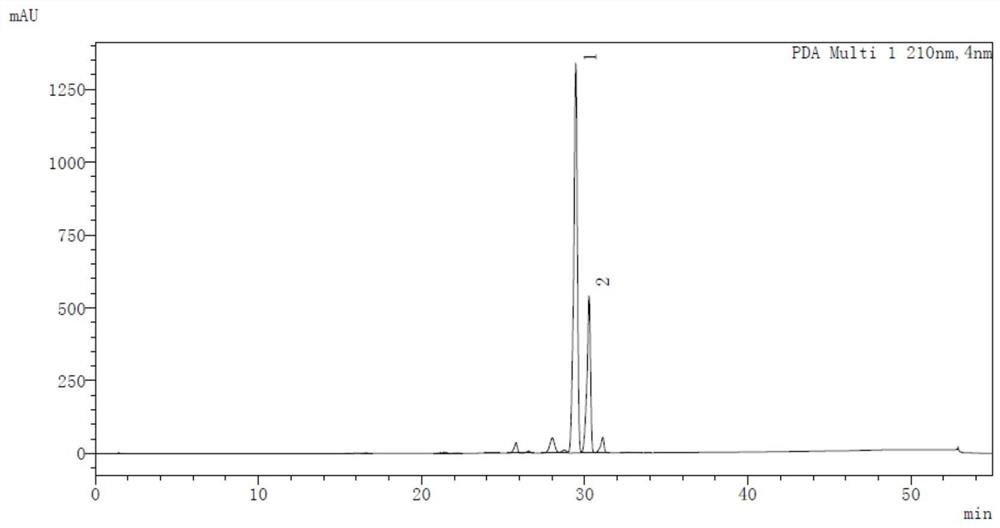
A kind of preparation method of 10-methoxy-4h-benzo[4,5] cycloheptatriene[1,2-b]thiazole-4-one
ActiveCN104817532BEasy to operateEasy post-processingOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsRefluxThiazole
The present invention discloses a 10-methoxy-4H-benzo[4,5]cycloheptatriene[1,2-b]thiazol-4-one preparation method, which comprises: mixing a raw material 9,10-dihydro-9,10-dibromo-4H-benzo[4,5]cycloheptatriene[1,2-b]thiazol-4-one and absolute methanol, carrying out stirring reflux for 2-6 h, adding a solid alkali catalyst to the reaction solution, continuously carrying out the reflux reaction for 2-6 h, carrying out separation purification on the reaction solution after completing the reaction, and recovering the solid alkali catalyst so as to obtain the product 10-methoxy-4H-benzo[4,5]cycloheptatriene[1,2-b]thiazol-4-one. According to the present invention, the solid alkali catalysis is used, the technology is easy to operate, the equipment is not corroded, the three-waste is less, the post-treatment is easy, the solid alkali can be reused, and the preparation method is the economical and practical green environmental protection technology.
Owner:湖州优研知识产权服务有限公司
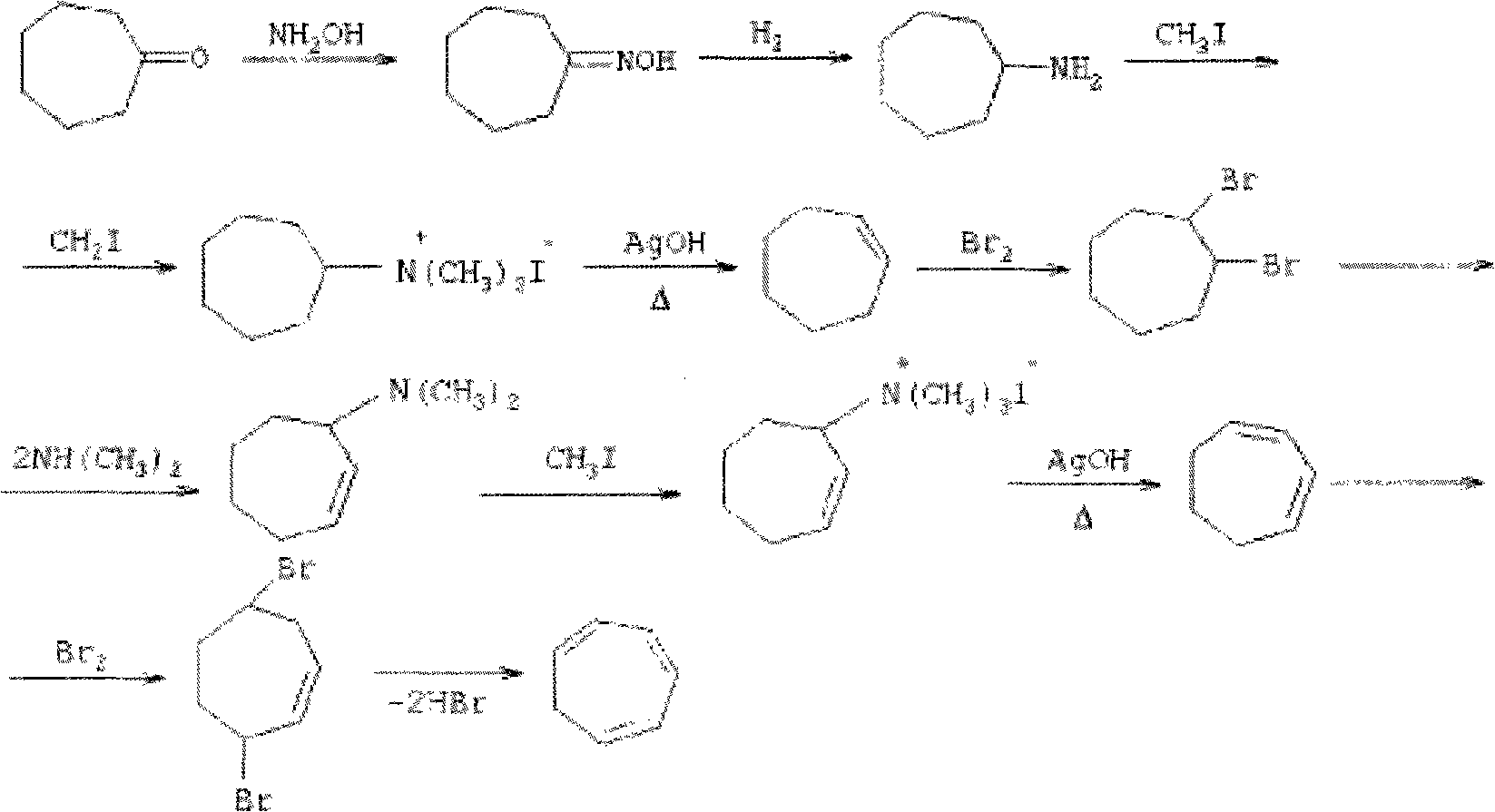
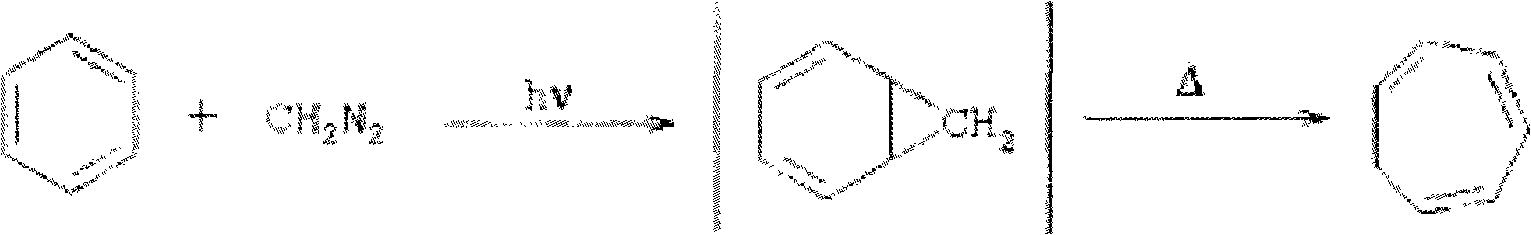
Method for synthesizing cycloheptatriene
InactiveCN101857521AShort reaction pathShort reaction timeHydrocarbon from halogen organic compoundsOrganic solventRoom temperature
The invention discloses a method for synthesizing cycloheptatriene, which comprises the following preparation steps of: adding 7,7-dichloro-bicyclo [4.1.0] heptane, an organic solvent and a copper salt catalyst into a reaction vessel and reacting for 2-10 hours at the temperature of 110-180 DEG C; and then, cooling a reaction mixture to normal temperature, adding an entrainer which can form an azeotrope with the generated cycloheptatriene into the reaction mixture, then distilling out the azeotrope formed by the cycloheptatriene and the entrainer at normal pressure, afterwards adding water into the azeotrope, then adding diethyl ether for extraction, extracting the obtained diethyl ether layer, drying, afterwards decompressing and distilling off the diethyl ether so as to obtain the cycloheptatriene. The method for synthesizing the cycloheptatriene has the advantages of mild reaction condition, short reaction route, short reaction period, simple postprocessing, high product yield, no use of raw materials with large toxicity, such as diazomethane and the like, better safety and environmental protection and is suitable for industrialized production.
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
A kind of negative ion environment-friendly adhesive based on acrylic resin and preparation method thereof
ActiveCN108276935BLow viscosityImprove stabilityNon-macromolecular adhesive additivesAcid polymer adhesivesPolymer scienceAcrylic resin
The invention discloses an anion environment-friendly adhesive based on acrylic resin and a preparation method of the anion environment-friendly adhesive. The anion environment-friendly adhesive is prepared by pre-polymerizing the following components in parts by weight until the viscosity reaches 1000 to 30000mpa.s, then rapidly cooling and stopping polymerizing. The anion environment-friendly adhesive is prepared from the following components: 30 to 300 parts of acrylic monomer or methacrylic monomer, 0 to 300 parts of other free radical polymeric monomers without carboxylic acid groups, 1 to 60 parts of negative oxygen ion powder, 0.1 to 5 parts of an initiator for free radical polymerization, 0.1 to 5 parts of cycloheptatriene phenolic acetone copper salt and 0.1 to 10 parts of a dispersing agent. The negative oxygen ion powder is coated with carboxyl on the acrylic resin monomer to form a micelle with a core-shell structure; the micelle is heated to be pre-polymerized to form theenvironment-friendly adhesive with proper viscosity; the negative oxygen ion powder positioned on the core of the micelle endows the environment-friendly adhesive with a negative oxygen ion release function, so that the inhibition of formaldehyde release can be realized. The cycloheptatriene phenolic acetone copper salt is an efficient broad-spectrum antibacterial agent and can be used for inhibiting the growth of most harmful microorganisms in the living environment and realizing the functions of preventing mildew and resisting viruses.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
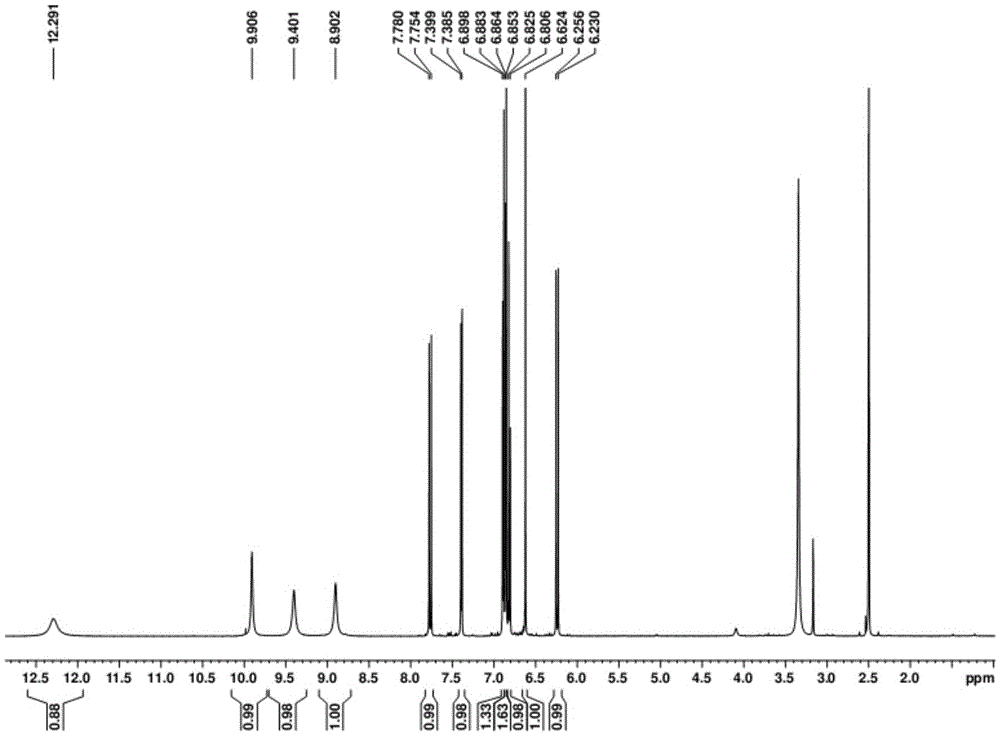
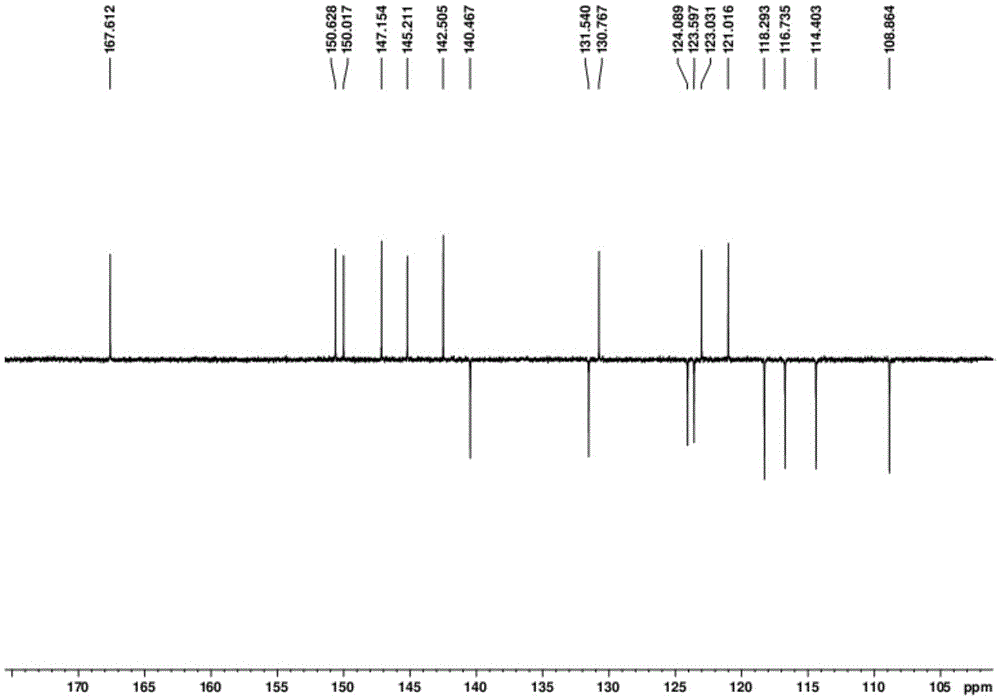
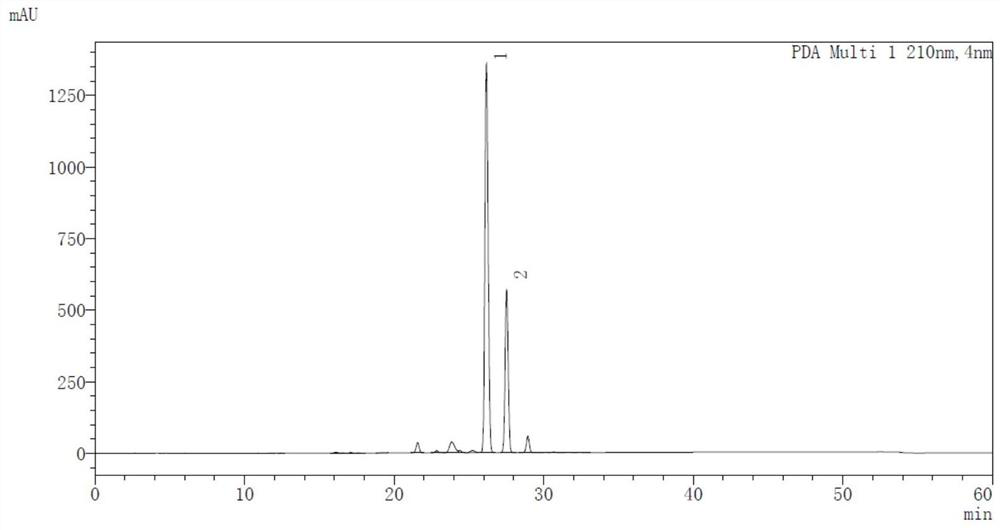
Preparation method of 5H-dibenzo[a,d]cycloheptatriene-5-one
PendingCN113929566AShort process routeSimple and fast operationOrganic compound preparationChemical/physical/physico-chemical microreactorsPtru catalystPhenacyl
The invention belongs to the technical field of drug synthesis, and provides a novel preparation method of 5H-dibenzo[a,d]cycloheptatriene-5-one. According to the invention, 3-(2-benzoylphenyl)acrylic acid is used as a starting material, and intramolecular decarboxylation coupling is performed under the action of a silver catalyst and an oxidizing agent to prepare a target product. The method has the characteristics of simplicity and convenience in operation, no need for isolating water and air, mild reaction conditions, high reaction rate and the like, and the product obtained by the method is relatively high in yield and purity and is more suitable for industrial production.
Owner:LUNAN PHARMA GROUP CORPORATION
High-elasticity acrylic ester waterproof coating preparation method
InactiveCN107964069AIncrease elasticityImprove waterproof performanceCoatingsLactone formationHydroxy compound
The invention provides a high-elasticity acrylic ester waterproof coating preparation method. The method includes steps: adding, by mass, 20-45 parts of elastic monomer, 15-35 parts of acrylic dodecylalcohol, 0.01-0.1 part of 5H-dibenzo[A,D]cycloheptatriene-5-ol, 0.001-0.01 part of 4-methyl-4-hydroxyl-5-hexenoic acid-gamma-lactone and 5-10 parts of acrylic acid into a reaction kettle with 20-30 parts of water and 0.5-2.5 parts of an emulsifying agent, and stirring for 0.5-1.5h to obtain pre-emulsified liquid for standby application; adding water and the emulsifying agent, then adding an initiator, slowly adding residual pre-emulsified liquid neutralized initiator mixed liquid into the reaction kettle after thermal reaction, finishing dropwise adding in 3-4h, then heating to perform reaction for 2-4h, cooling to 30-40 DEG C, and adjusting a pH value to 6-8 to obtain high-elasticity acrylic ester coating.
Owner:王琪宇
Precursors and methods for atomic layer deposition of transition metal oxides
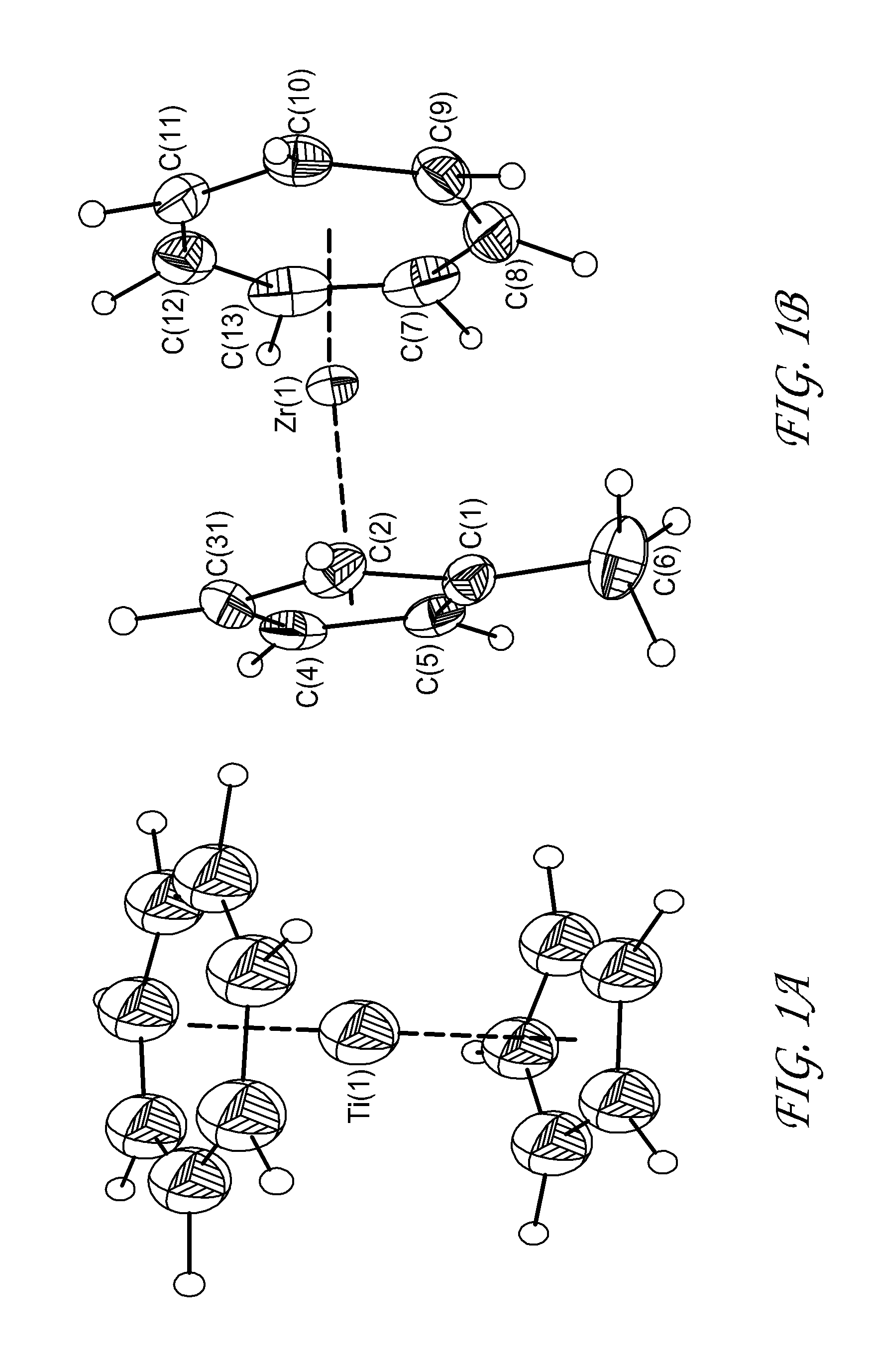
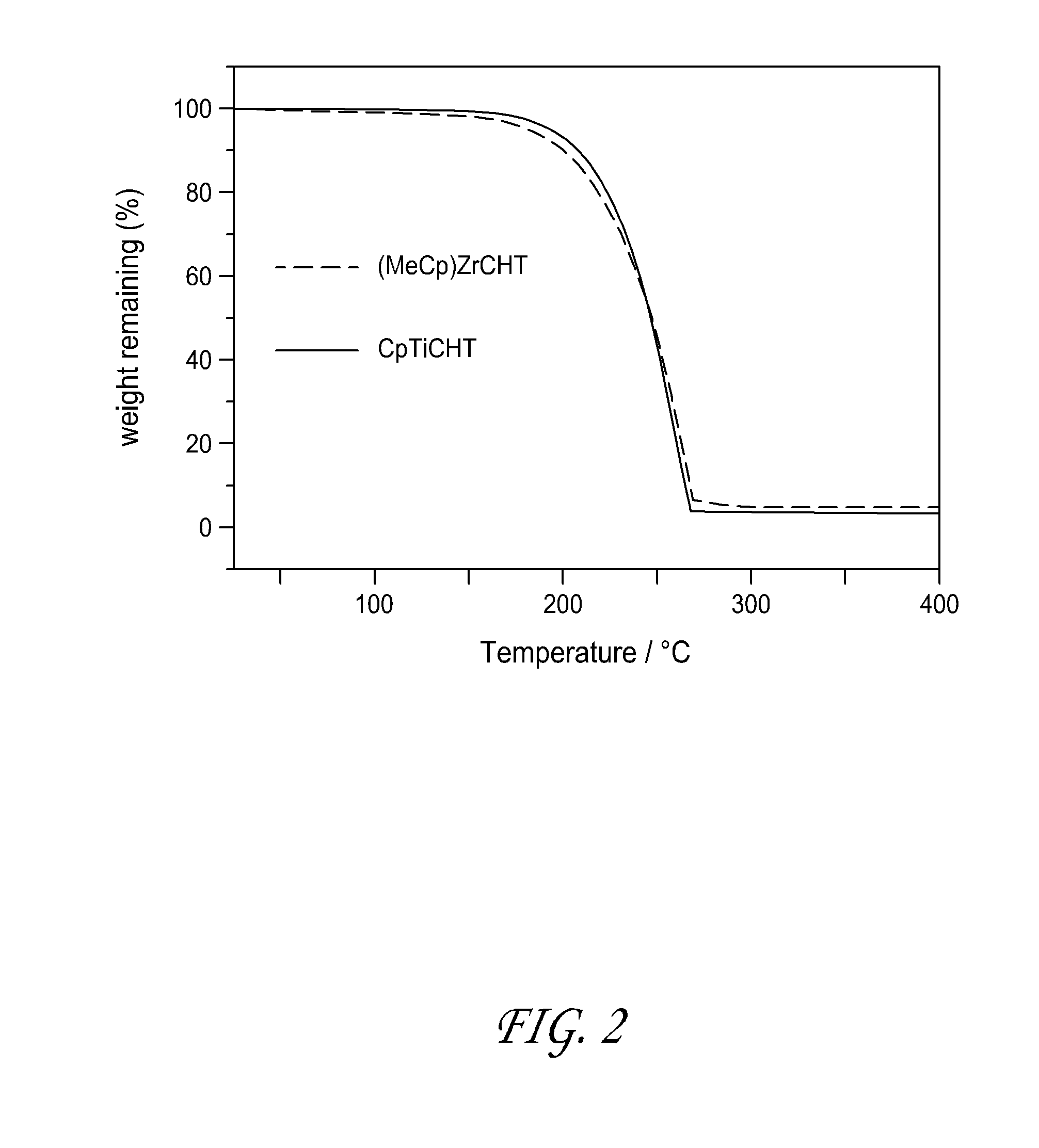
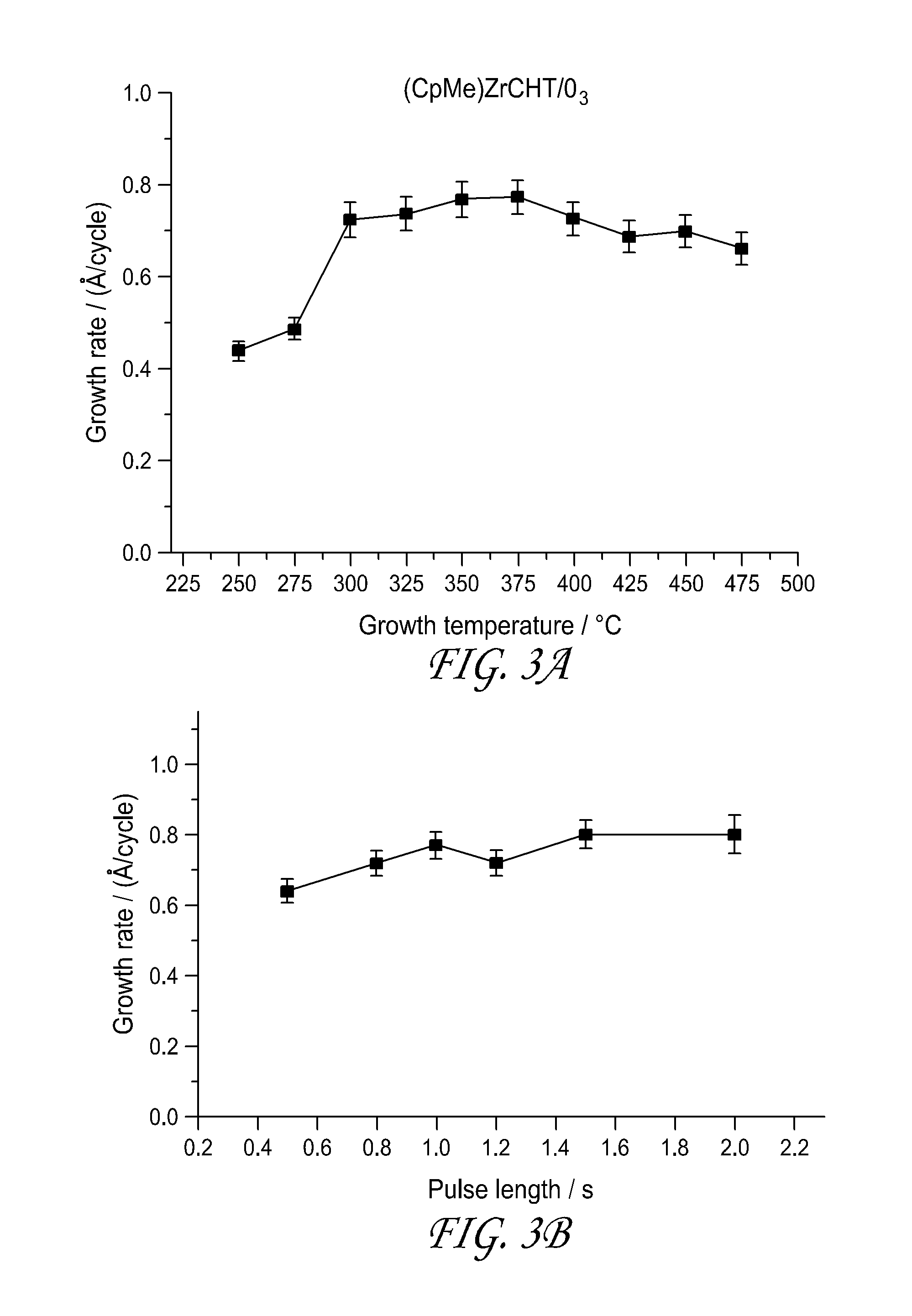
ActiveUS20150191817A1Group 4/14 element organic compoundsTitanium dioxideAtomic layer depositionCapacitor
Methods are provided herein for forming transition metal oxide thin films, preferably Group IVB metal oxide thin films, by atomic layer deposition. The metal oxide thin films can be deposited at high temperatures using metalorganic reactants. Metalorganic reactants comprising two ligands, at least one of which is a cycloheptatriene or cycloheptatrienyl (CHT) ligand are used in some embodiments. The metal oxide thin films can be used, for example, as dielectric oxides in transistors, flash devices, capacitors, integrated circuits, and other semiconductor applications.
Owner:ASM INTERNATIONAL
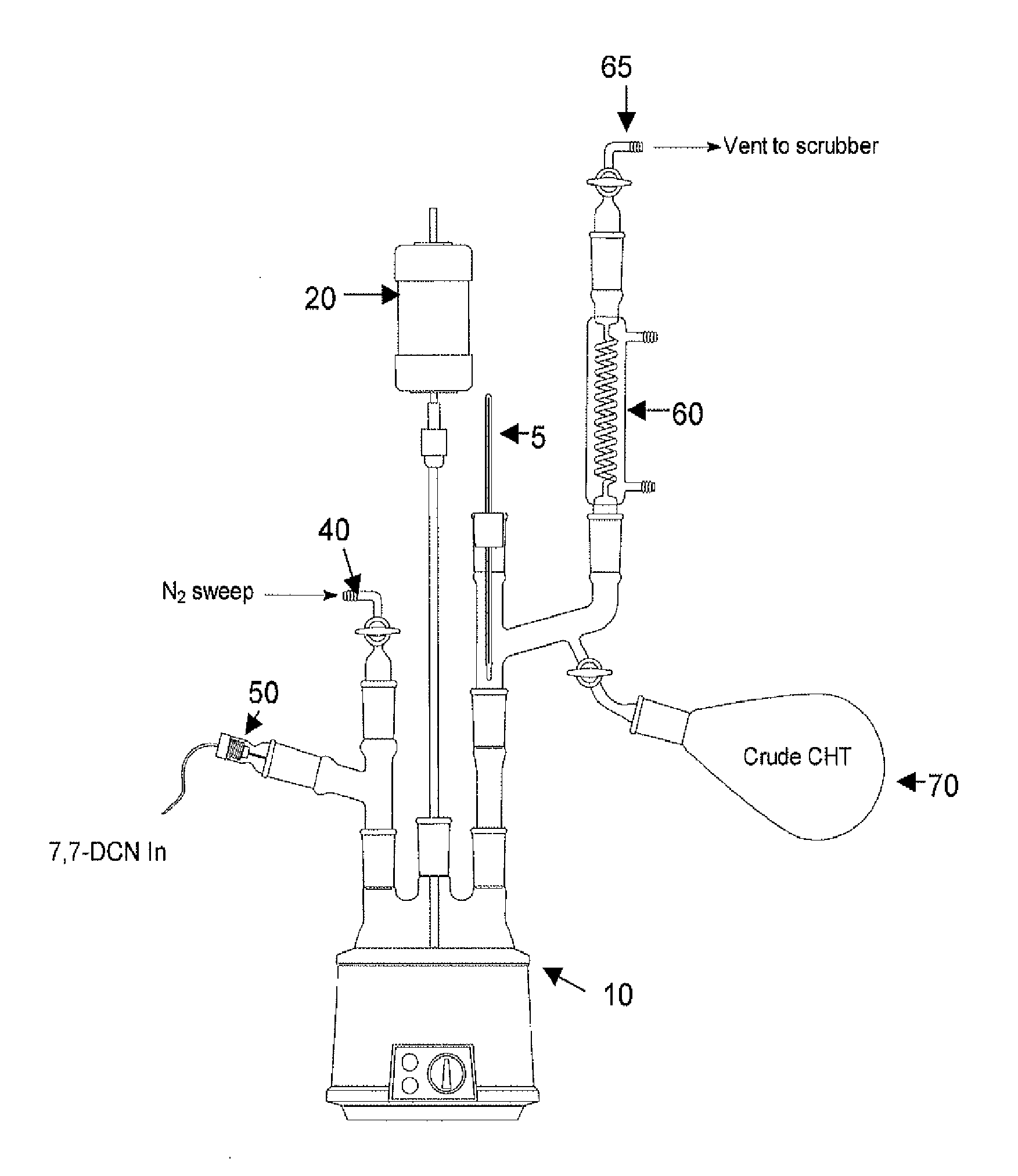
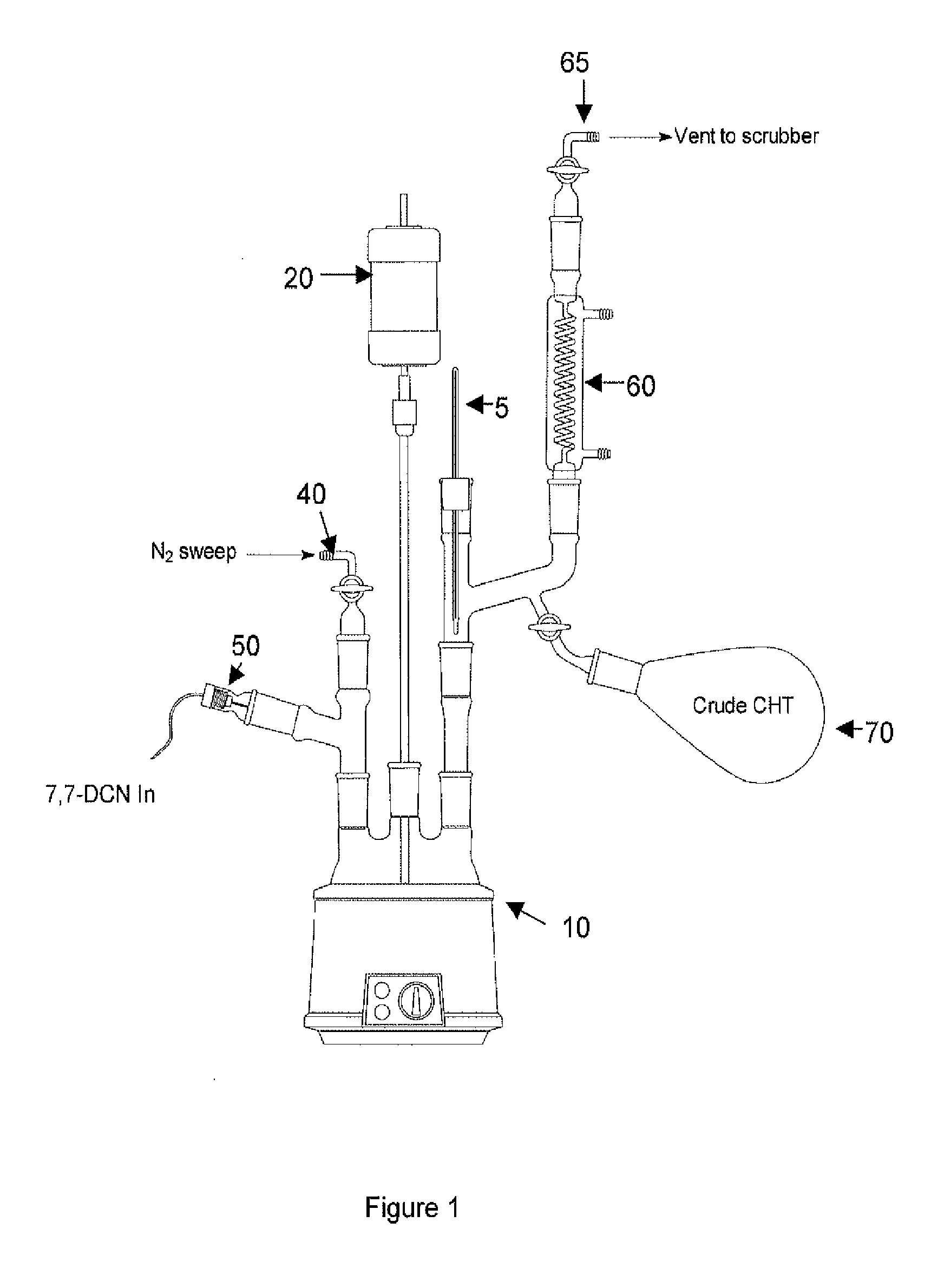
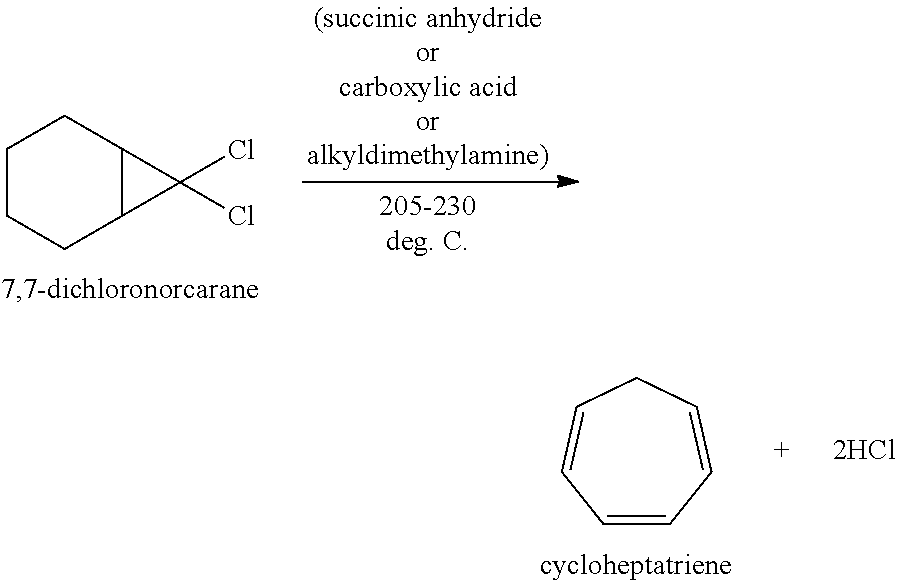
Methods for producing cycloheptatriene
ActiveUS20130066127A1Satisfies needHydrocarbon from oxygen organic compoundsHydrocarbon from halogen organic compoundsCarboxylic acidSuccinic anhydride
This invention relates to methods for producing cycloheptatriene from at least 7,7-dichloronorcarane and a liquid component comprising a C8 to C30 succinic anhydride, a carboxylic acid, or a C8 to C30 alkyldimethylamine at about 205 deg. C. to about 230 deg. C.
Owner:WR GRACE & CO CONN
A kind of preparation method of multi-substituted cycloheptatriene derivative
ActiveCN109369672BThe synthesis method is scientific and reasonableThe synthesis method is simpleOrganic chemistryRotary evaporatorOrganic synthesis
The invention discloses a preparation method of multi-substituted cycloheptatriene derivatives, which belongs to the technical field of organic synthesis. The method is as follows: in the reactor, add substituted N-alkylbenzamide and iodobenzene diacetate. After the reaction was stirred in the solvent, the filtrate was concentrated using a rotary evaporator to obtain a crude product, which was separated by silica gel column chromatography to obtain the target compound. The synthesis method of the multi-substituted cycloheptatriene derivative provided by the invention has the characteristics of scientific rationality, simple synthesis method, high yield of the target compound, easy purification of the product, fast reaction, and room temperature reaction temperature. Its reaction equation is as follows:
Owner:QINGDAO UNIV OF SCI & TECH
Polysubstituted difuro-cycloheptatrienamine derivative and preparation method thereof
The invention discloses a polysubstituted difuro-cycloheptatrienamine derivative and a preparation method thereof. The structural general formula of the polysubstituted difuro-cycloheptatrienamine derivative is as shown in formula I, in the formula I, R is selected from one of aryl, fused aryl and alkyl, R1 is selected from one of aryl and fused aryl, R2 is selected from alkyl, R3 is shown in the specification, and R4 is selected from one of alkyl and alkoxy. The preparation method comprises the following steps: taking an isonitrile compound and a conjugated eneyne ketone compound as raw materials, and carrying out cycloaddition cascade reaction under the promotion condition of zinc chloride to directly generate the furo-cycloheptatriene derivative. The preparation method has the advantages of simple and mild reaction conditions, wide and easily available raw material range, high atom utilization rate, simplicity and convenience in operation and the like.
Owner:SHANDONG NORMAL UNIV
Tyrosine kinase inhibitors
ActiveCN101535268BAlleviate time dependenceOrganic active ingredientsSenses disorderMedicineTyrosine-kinase inhibitor
The present invention relates to 5H-benzo[4,5]cyclohepta[ 1,2-b]pyridine derivatives of formula (I) that are useful for treating cellular proliferative diseases, for treating disorders associated with MET activity, and for inhibiting the receptor tyrosine kinase MET. The invention also related to compositions which comprise these compounds, and methods of using them to treat cancer in mammals.
Owner:MERCK SHARP & DOHME BV
3-methyl-octahydro-cycloheptatriene oxapicene-2-ketone and preparation method thereof
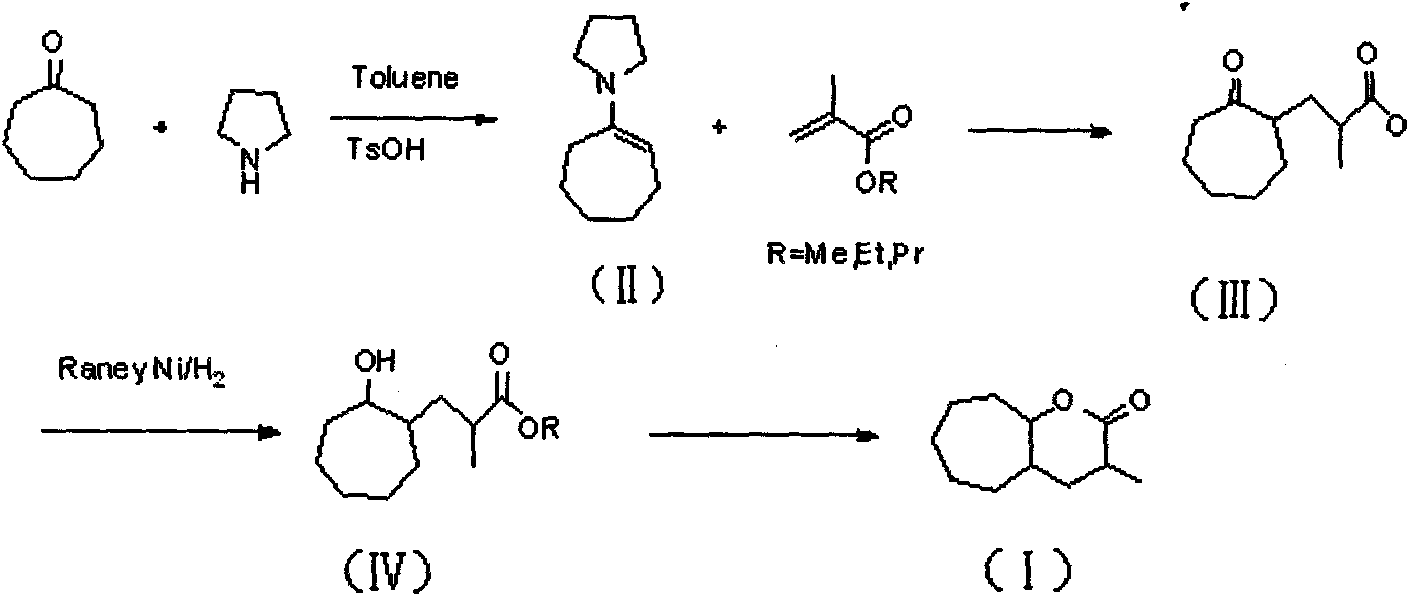
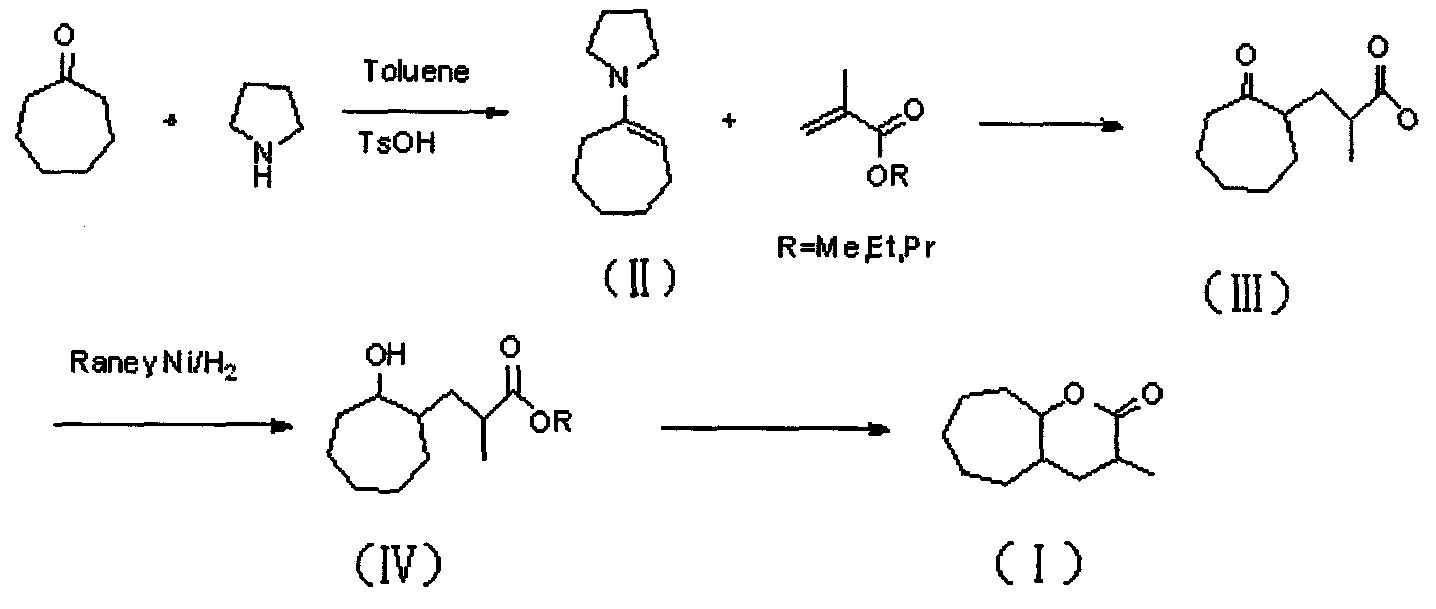
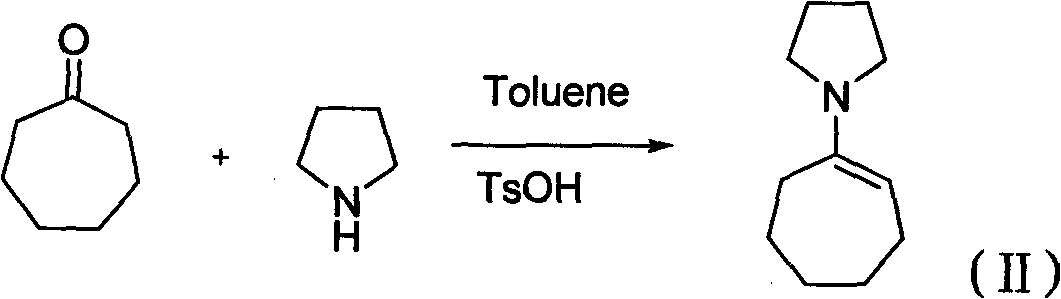
InactiveCN101774998ANo hepatotoxicityRaw materials are cheap and easy to getOrganic chemistryFood preparationAlcoholEnamine
The invention discloses 3-methyl-octahydro-cycloheptatriene oxapicene-2-ketone and a preparation method thereof. The preparation method of the 3-methyl-octahydro-cycloheptatriene oxapicene-2-ketone mainly comprises the steps of the preparation of cycloheptyl pyrrole enamine, the preparation of 2-methyl ester-cycloheptane ketone, the preparation of methyl ester-cycloheptane alcohol, the preparation of 3-methyl-octahydro cycloheptatriene oxapicene and the like. The preparation method of the 3-methyl-octahydro-cycloheptatriene oxapicene-2-ketone has the advantages of low cost, easy implementation of reaction conditions, high reaction yield and stable product quality, and is convenient for industrialized production. The 3-methyl-octahydro-cycloheptatriene oxapicene-2-ketone prepared through the invention has tonka-bean smell and is suitable for preparing tonka-bean lactone essences.
Owner:SHANGHAI INST OF TECH +1
A kind of polysubstituted furanocycloheptatriene pyrrole derivative and preparation method thereof
The invention discloses a polysubstituted furanocycloheptatriene pyrrole derivative and a preparation method thereof. a kind of; R 1 Aryl; R 2 is an alkyl group; R 3 is an acyl group. The preparation method is as follows: using isonitrile compounds and conjugated enynone compounds under the condition jointly promoted by zinc chloride and m-chloroperoxybenzoic acid, a cycloaddition series reaction is carried out to directly generate furanocycloheptatriene pyrrole derivatives The preparation method has the advantages of simple and mild reaction conditions, a wide range of substrates and easy availability, high atom utilization, and simple operation.
Owner:SHANDONG NORMAL UNIV
Adsorbent for purification of tribromostyrene
InactiveCN106167534AImprove thermal stabilityLarge specific surface areaOther chemical processesHalogenated hydrocarbon preparationPolyvinyl alcoholSorbent
The invention provides a preparation method of an adsorbent for purification of tribromostyrene. The preparation method comprises the following steps: dispersing a certain amount of vinylidene fluoride into water, adding 10-Undecenyldimethylchlorosilane, 3 methyl 1,5 di-p-toluene 1,4 pentazdiene, ammonium persulfate and polyvinyl alcohol, divinyl adipate, 2-hydroxyl 4 isopropyl 2,4,6 cycloheptatriene 1 one and 5 methylene 2 norbornene in proportion, heating, reacting at a preference temperature, filtering a product after the reaction, and drying so as to obtain the adsorbent for purification of tribromostyrene.
Owner:王琪宇
Method for producing propylene oxide
InactiveUS20100197945A1Efficient productionOrganic chemistryBulk chemical productionCyclopenteneOrtho position
There is disclosed a method for producing propylene oxide, which includes: reacting propylene, oxygen, and hydrogen in the presence of a noble metal catalyst and a titanosilicate catalyst in a liquid phase containing a polycyclic compound, which is unsubstituted or substituted with at least one substituent selected from Group B below, wherein the polycyclic compound is composed of two or more identical or different ring compounds selected from Group A below and the ring compounds are fused, directly bonded, or bonded by a linkage group selected from the group consisting of an oxygen atom, carbon chain, and a group composed of oxygen atom(s) and a carbon chain, provided that said polycyclic compound is not a polycyclic compound having hydroxy groups or oxo groups at para or. ortho positions. Group A consisting of benzene, cyclopentadiene, cycloheptatriene, furane, pyrane, cyclopentene, cyclopentane, cyclohexane, cyclohexene, cyclohexadiene, cycloheptane, cycloheptene, and cycloheptadiene. Group B consisting of halogen atom, alkyl group, alkenyl group, alkoxy group, hydroxyalkyl group, acyl group, oxo group, hydroxy group, and cyano group.
Owner:SUMITOMO CHEM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![A kind of preparation method of 10-methoxy-4h-benzo[4,5] cycloheptatriene[1,2-b]thiazole-4-one A kind of preparation method of 10-methoxy-4h-benzo[4,5] cycloheptatriene[1,2-b]thiazole-4-one](https://images-eureka.patsnap.com/patent_img/086a3625-701b-4172-bfdf-79aabaaff8df/BDA0000677408260000011.png)
![A kind of preparation method of 10-methoxy-4h-benzo[4,5] cycloheptatriene[1,2-b]thiazole-4-one A kind of preparation method of 10-methoxy-4h-benzo[4,5] cycloheptatriene[1,2-b]thiazole-4-one](https://images-eureka.patsnap.com/patent_img/086a3625-701b-4172-bfdf-79aabaaff8df/BDA0000677408260000021.png)
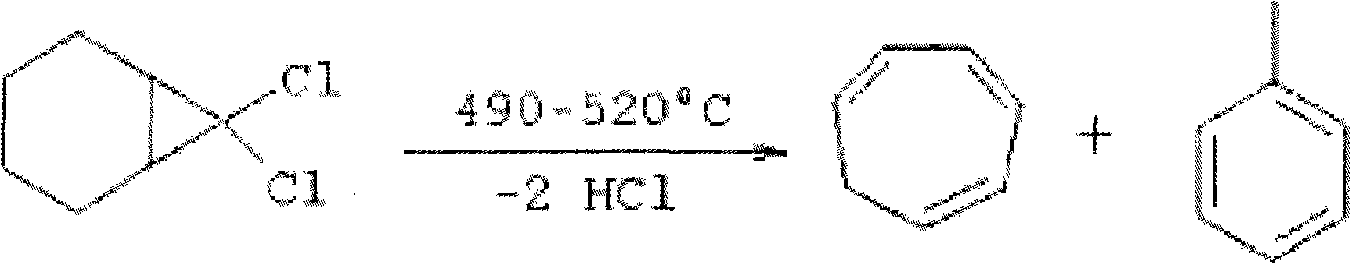
![Preparation method of 5H-dibenzo[a,d]cycloheptatriene-5-one Preparation method of 5H-dibenzo[a,d]cycloheptatriene-5-one](https://images-eureka.patsnap.com/patent_img/48aac919-4d1e-4658-a3cf-fd24580bc180/FDA0002560259770000011.png)
![Preparation method of 5H-dibenzo[a,d]cycloheptatriene-5-one Preparation method of 5H-dibenzo[a,d]cycloheptatriene-5-one](https://images-eureka.patsnap.com/patent_img/48aac919-4d1e-4658-a3cf-fd24580bc180/BDA0002560259780000011.png)
![Preparation method of 5H-dibenzo[a,d]cycloheptatriene-5-one Preparation method of 5H-dibenzo[a,d]cycloheptatriene-5-one](https://images-eureka.patsnap.com/patent_img/48aac919-4d1e-4658-a3cf-fd24580bc180/BDA0002560259780000012.png)