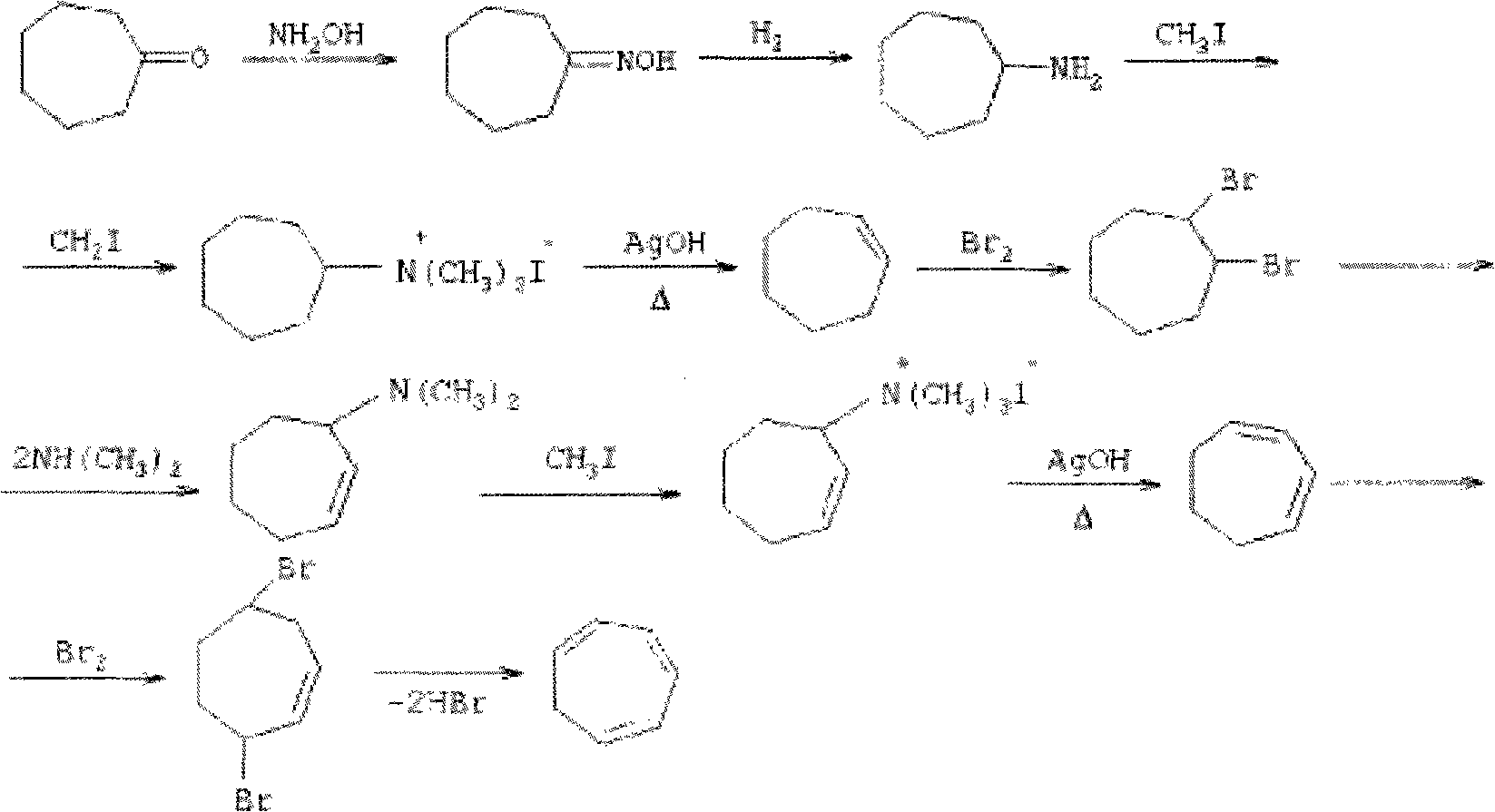
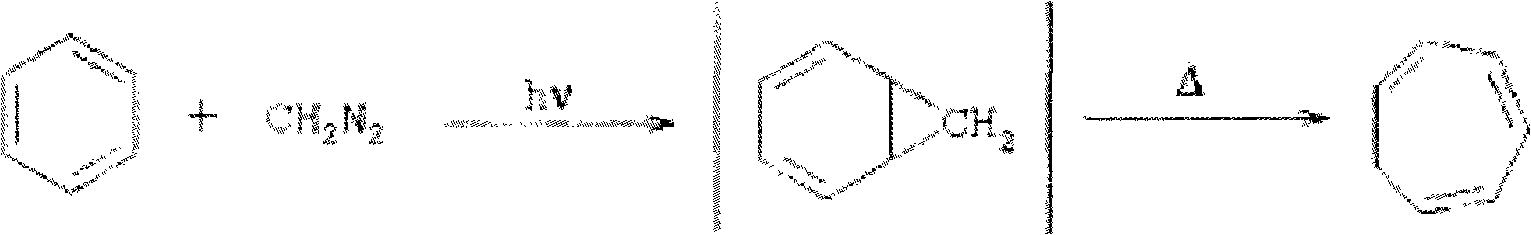
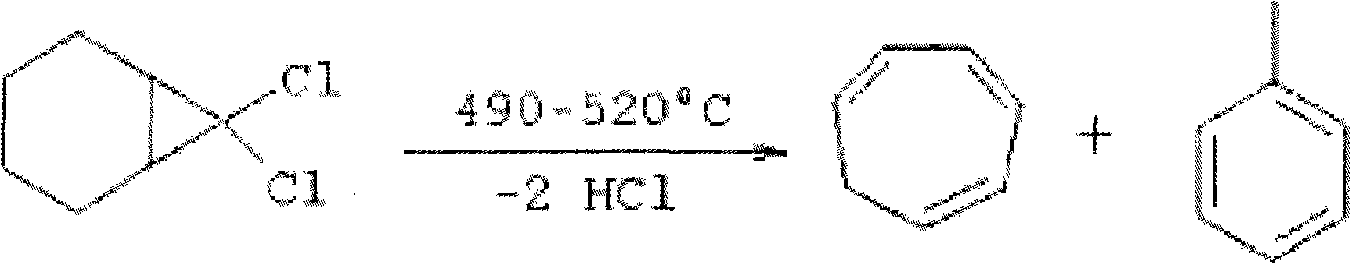Method for synthesizing cycloheptatriene
A technology of cycloheptatriene and a synthesis method is applied in the fields of pharmaceutical intermediates, medicines, chemical intermediates and pesticides, and can solve the problems of high toxicity of synthetic raw material diazomethane, unsuitable for large-scale industrial production, unsuitable for industrialized production and the like , to achieve the effect of ensuring safety, low toxicity and saving reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add 165 grams (1.0mol) 7,7-dichlorobicyclo[4.1.0] heptane in 1000 milliliters one-mouth bottle, 400g (3.7mol) diethylene glycol (molecular weight is 106 polyethylene glycols), 20 grams (0.2 mol) of cuprous chloride was stirred and reacted at 140° C. for 8 hours. Then above-mentioned reaction mixture is cooled to normal temperature, adds 300 milliliters of methanol, distills out the azeotrope (cut) of methanol and cycloheptatriene at 80 ℃, transfers azeotrope in the separatory funnel, adds 200 milliliters of water, then Extracted twice with 150 ml of ether, combined the ether layers, dried, and distilled off the ether under reduced pressure to obtain 45 g of oily liquid cycloheptatriene. Yield 50%.
Embodiment 2
[0029] Add 165 grams (1.0mol) of 7,7-dichlorobicyclo[4.1.0]heptane, 500 grams of PEG600 (0.83mol), 15 grams (0.1mol) of cuprous bromide in a 1000 milliliter single-necked bottle, and stir at 140°C React for 8 hours. Cool to normal temperature, add 300 ml of methanol, distill out the azeotrope of methanol and cycloheptatriene at 80°C, transfer the azeotrope to a separatory funnel, add 200 ml of water, extract twice with 200 ml of ether, combine the ether layers, and dry , Diethyl ether was distilled off under reduced pressure to obtain 28 grams of oily liquid cycloheptatriene. Yield 32%.
Embodiment 3
[0031] Add 165 grams (1.0mol) of 7,7-dichlorobicyclo[4.1.0]heptane, 500 grams of PEG2000 (0.25mol), 15 grams (0.1mol) of cuprous bromide in a 2000 milliliter single-necked bottle, and stir at 140°C React for 10 hours. Cool to normal temperature, add 500 ml of methanol, distill out the azeotrope of methanol and cycloheptatriene at 80°C, transfer the azeotrope to a separatory funnel, add 400 ml of water, extract twice with 300 ml of ether, combine the ether layers, and dry , Diethyl ether was distilled off under reduced pressure to obtain 42 g of oily liquid cycloheptatriene. Yield 46.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com