Patents
Literature
34 results about "Cycloheptanone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cycloheptanone, (CH₂)₆CO, is a cyclic ketone also referred to as suberone. It is a colourless volatile liquid. Cycloheptanone is used as a precursor for the synthesis of pharmaceuticals.
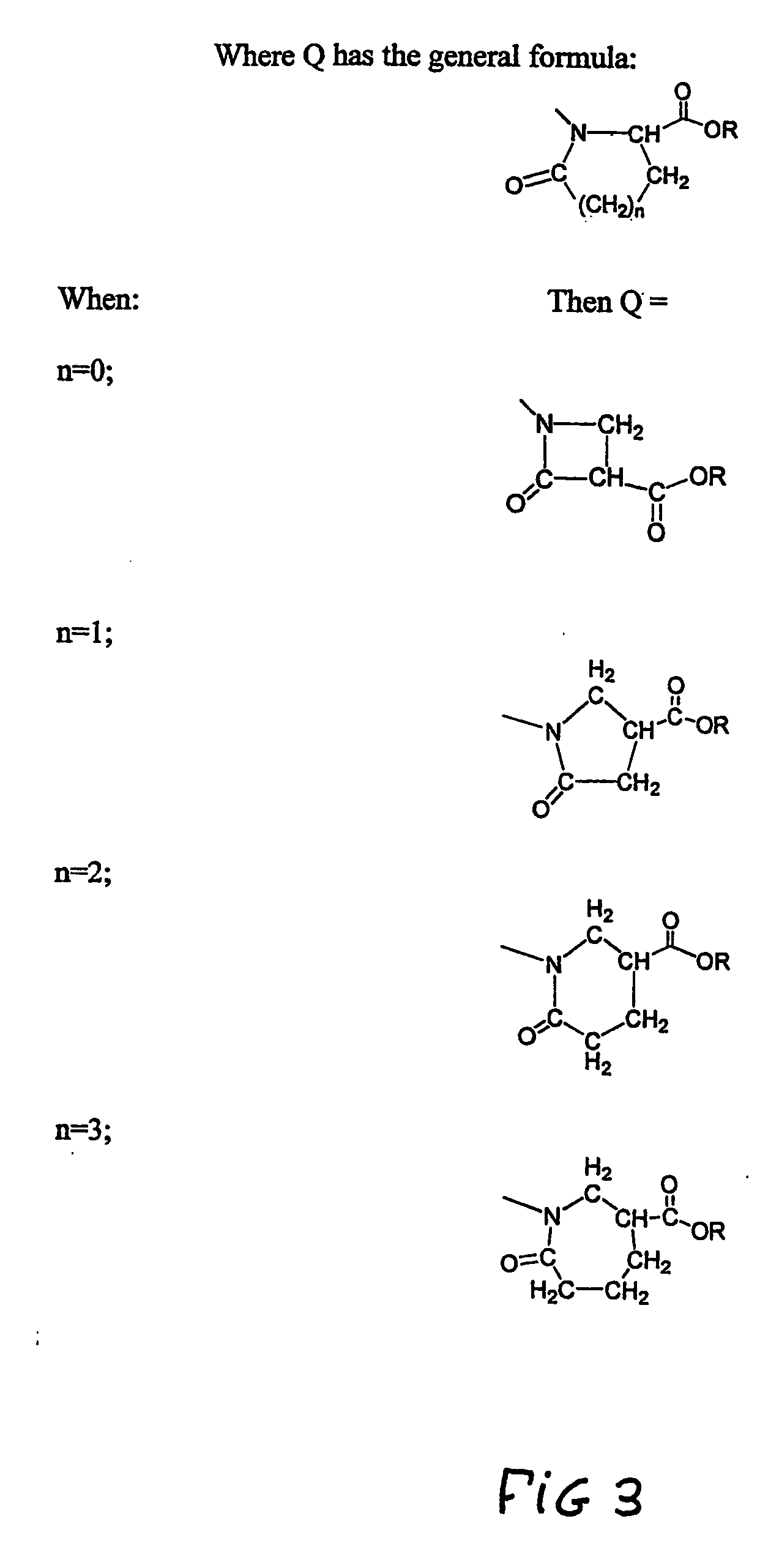
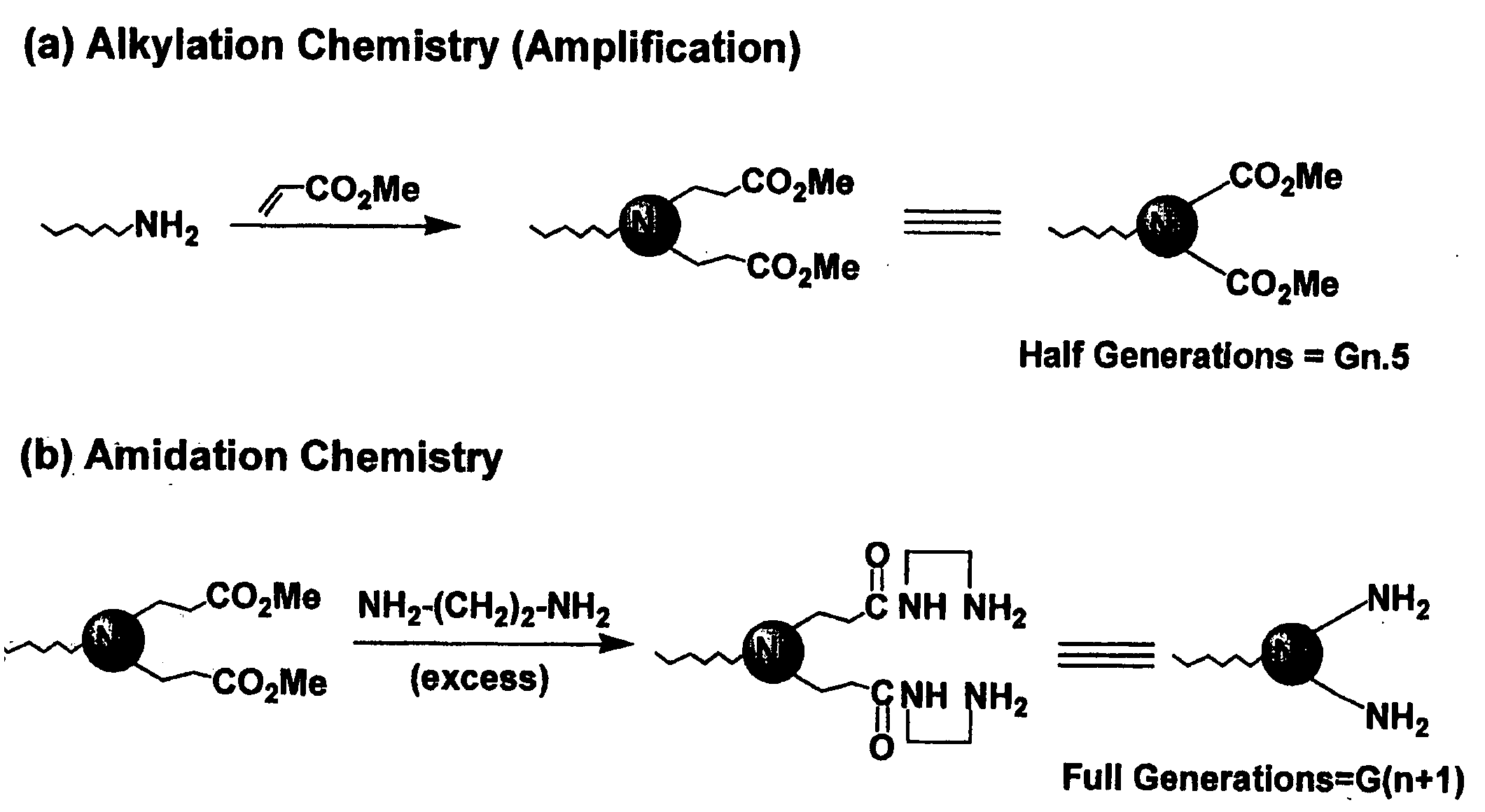
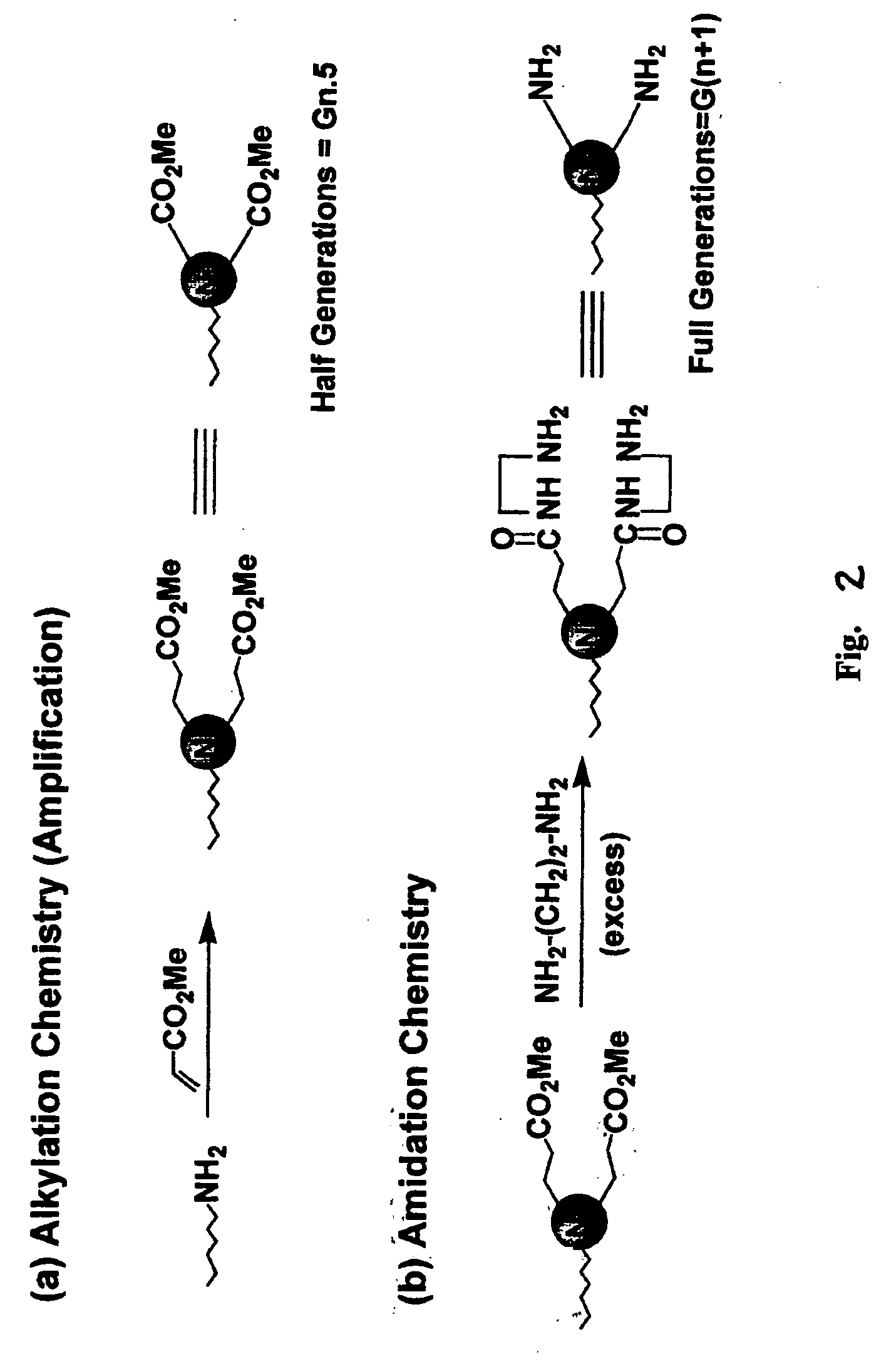
Heterocycle functionalized dendritic polymers
Heterocycle terminated dendritic polymers. More specifically, the production of 2-pyrrolidone, 2-piperidone, 2-aza-cycloheptanone or 2-azetidinone-terminated dendritic polymers obtained by reacting precursor primary amine, (e.g., —NH2)-terminated dendritic polymers with certain functionalized methacrylate reagents to produce new and novel dendritic polymers terminated with ester substituted 2-pyrrolidone, 2-piperidone, 2 aza-cycloheptanone or 2-azetidinone groups.
Owner:DENDRITIC NANO TECH INC
Pyrrolidone, piperidone and azetidinone terminated and functionalizes dendritic polymers
InactiveUS20050171298A1Synthetic resin layered productsThin material handlingMethacrylate2-Pyrrolidone
Heterocycle terminated dendritic polymers. More specifically, the production of 2-pyrrolidone, 2-piperidone, 2-aza-cycloheptanone or 2-azetidinone-terminated dendritic polymers obtained by reacting precursor primary amine,(e.g., —NH2)-terminated dendritic polymers with certain functionalized methacrylate reagents to produce new and novel dendritic polymers terminated with ester substituted 2-pyrrolidone, 2-piperidone, 2-aza-cycloheptanone or 2-azetidinone groups.
Owner:DENDRITIC NANO TECH INC
Starch / C4~C8 ring graft copolymer and prep. and use thereof
The present invention is starch / C4-C8 cyclic monomer grafted copolymer and its preparation process. Starch of corn, beans, potato and grains in grain size of 1-20 microns or modified starch in 1-60 portions and one or several C4-C8 cyclic monomer p-dioxy cyclohexanone, 1, 5-dioxy suberyl-2-one, gamma-butyrolactone, beta-butyrolactone, delta-valerolactone, epsilon-caprolactone, diglycolide and lactide in 40-99 portions react in a reaction bottle under the protection of inert gas and in the presence of catalyst in 0.01-5 portions at 60-150 deg.c for 10-48 hr to produce grafted copolymer. The unreacted monomer and homopolymer are extracted separately with solvent, the product is vacuum dried to constant weight, and the monomer converting rate, grafting rate and grafting efficiency are measured.
Owner:SICHUAN UNIV
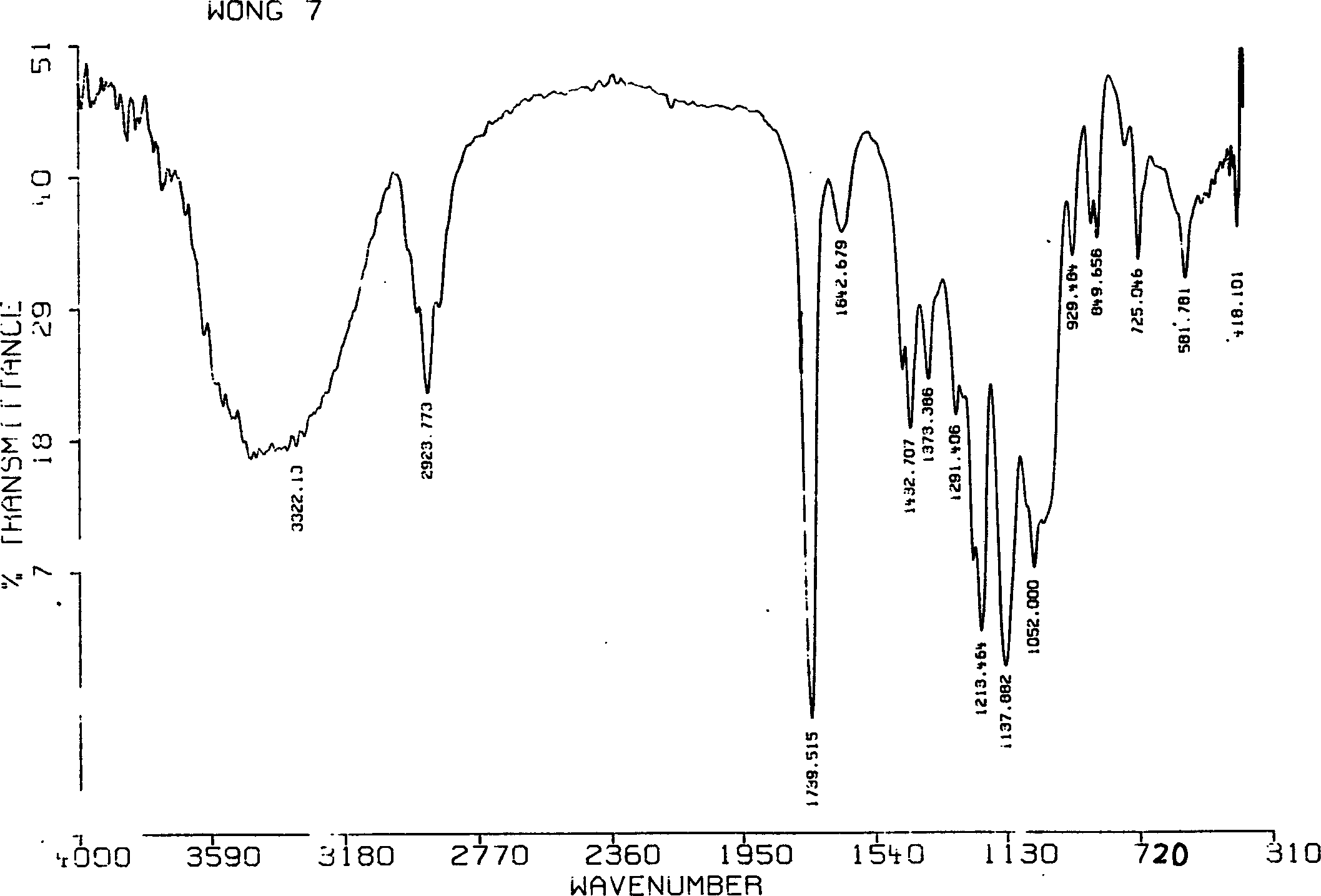
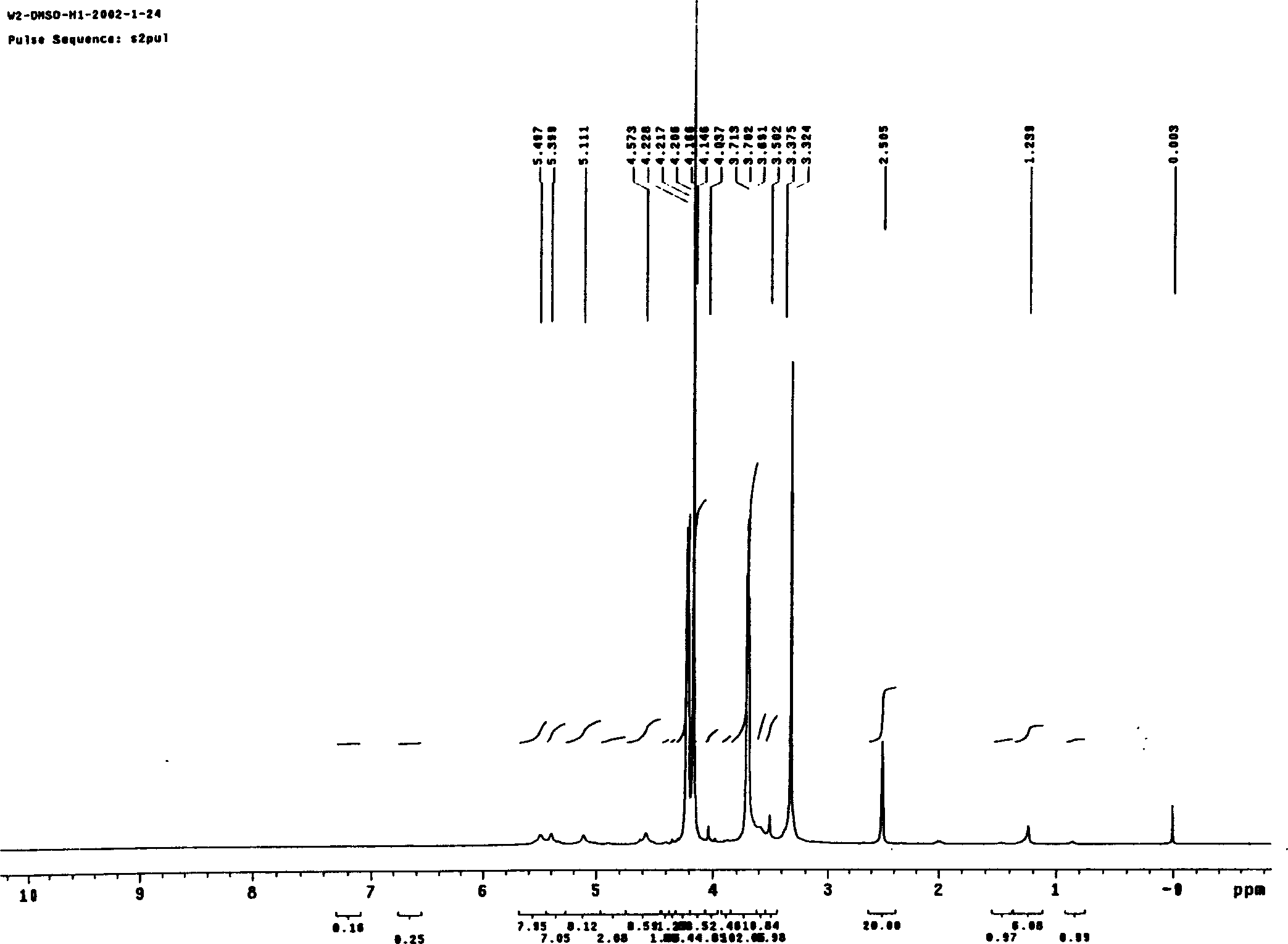
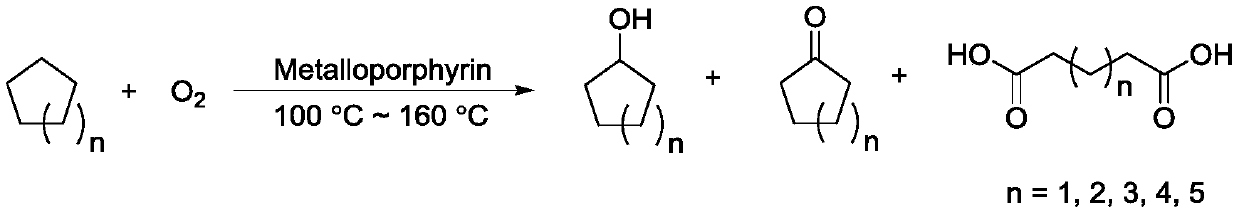
Cycloalkane catalytic oxidation method promoted by iron porphyrin
PendingCN110560169AReduce dosageHigh catalytic efficiencyPreparation by oxidation reactionsOrganic compound preparationAlkaneCyclononane
The invention relates to a cycloalkane catalytic oxidation method promoted by iron porphyrin. O2 is taken as an oxidant, Co (II) porphyrin and Mn (II) porphyrin are taken as main catalysts, and Fe (II) porphyrin is taken as a cocatalyst to carry out reacting at a temperature of 100-160 DEG C and pressure of 0.8-2.0MPa under a solvent-free condition to realize catalytic oxidation of cycloalkanes. The cycloalkanes mainly comprise cyclopentane, cyclohexane, cycloheptane, cyclooctane and cyclononane, and the corresponding oxidation products mainly comprise cycloalkyl alcohols, cycloalkyl ketones and alkyl diacids. The cycloalkane catalytic oxidation method has the advantages of high selectivity of cyclopentanone, cyclohexanol, cycloheptanone and cyclooctanone, small catalyst consumption and simple operation, and environmental friendliness is achieved by taking clean O2 as the oxidant. The disclosesd cycloalkane catalytic oxidation method promoted by iron porphyrin is a cycloalkane catalytic oxidation method with high product selectivity, simple operation and environmental friendliness.
Owner:ZHEJIANG UNIV OF TECH
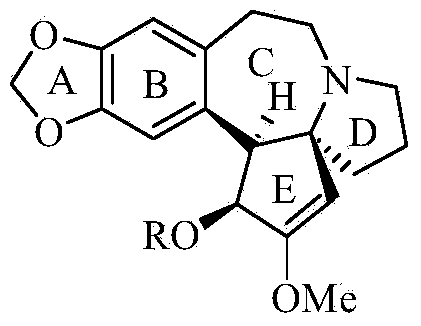
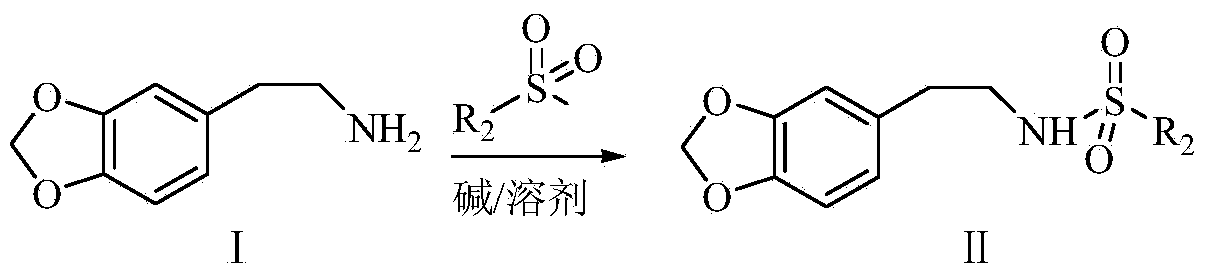
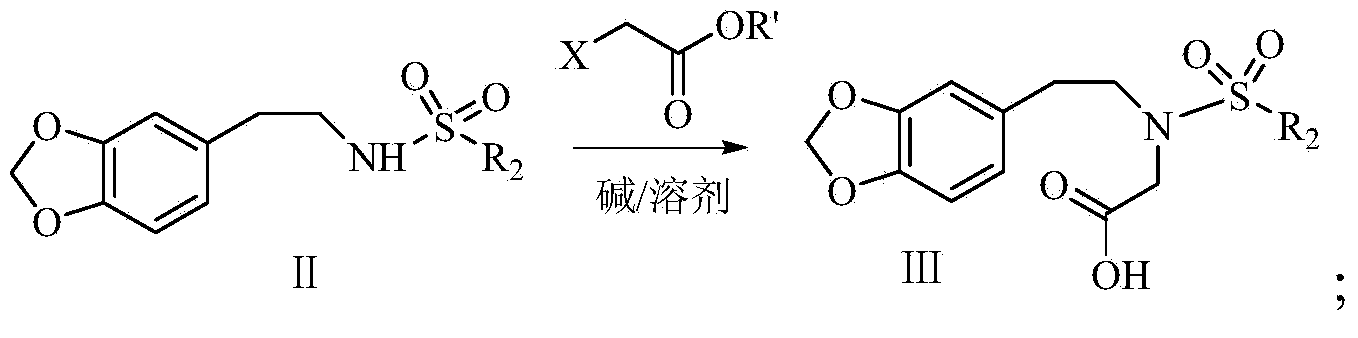
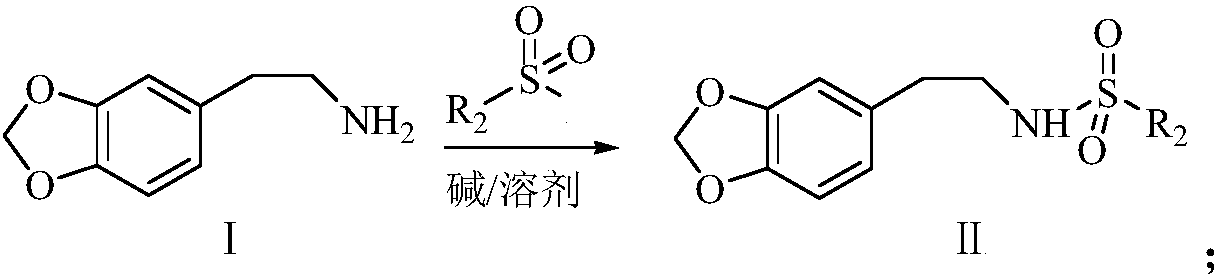
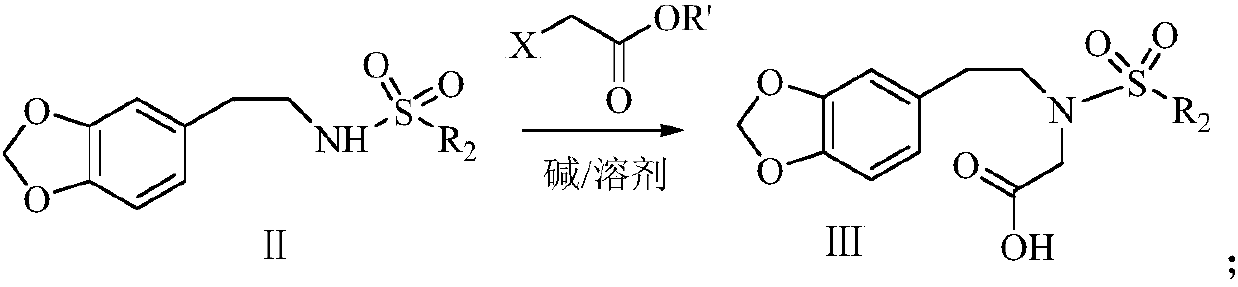
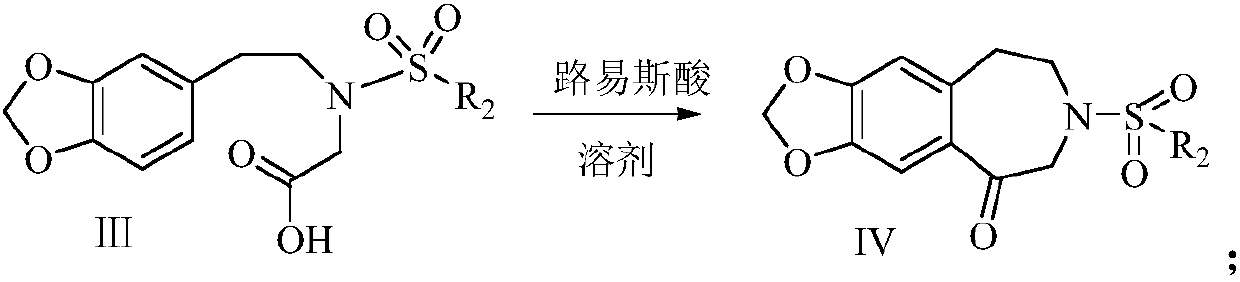
Synthetic method of harringtonine C-ring intermediate
The invention relates to an artificial synthetic method of a harringtonine C-ring intermediate. According to the method, 3,4-methylenedioxyphenethylamine (compound I) as a raw material is subjected to acylation in alkaline conditions to obtain N-acylated-3,4-methylenedioxyphenethylamine (compound II), which is then reacts with halogenated acetic acid or halogenated acetic acid derivative to generate N-acylated-3,4-methylenedioxyphenethylamine acetic acid (compound III); and the compound (III) is catalyzed by Lewis acid to generate intramolecular Friedel-crafts acylation reaction to obtain N-acyl-3,4-metheneoxybenzo-3-N-cycloheptanone, namely C ring of harringtonine. In the preparation process, products of each step are purified by washing and recrystallization; and the method has simple operation, total yield of 87.4%, and good industrial prospect.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
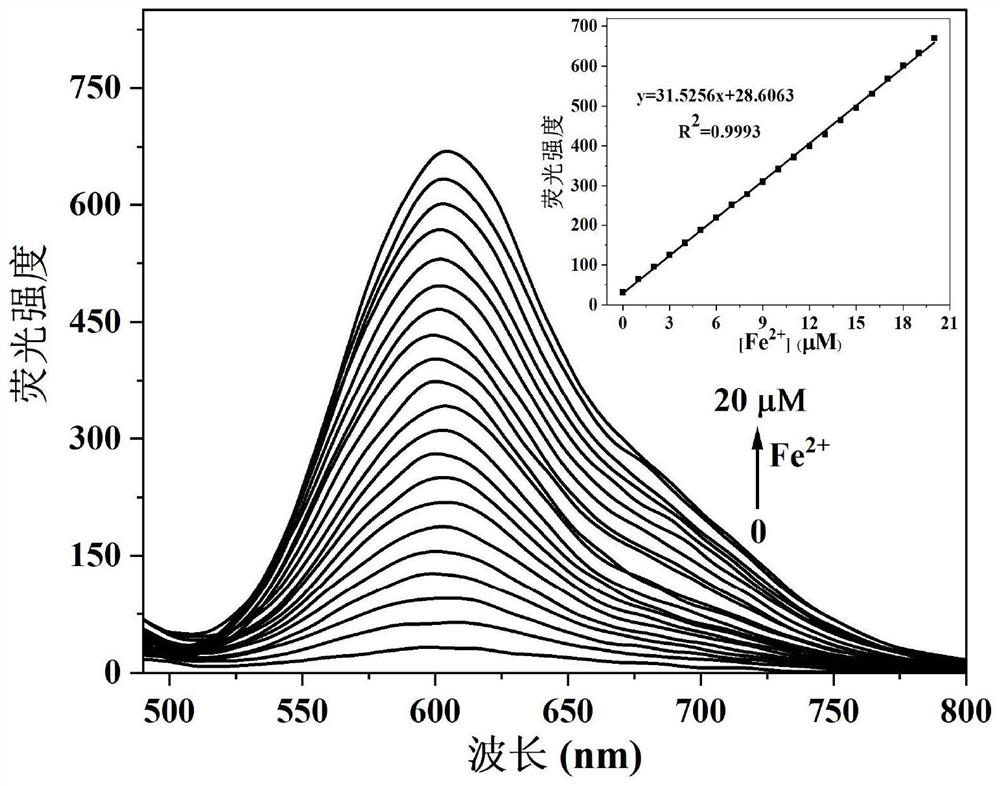
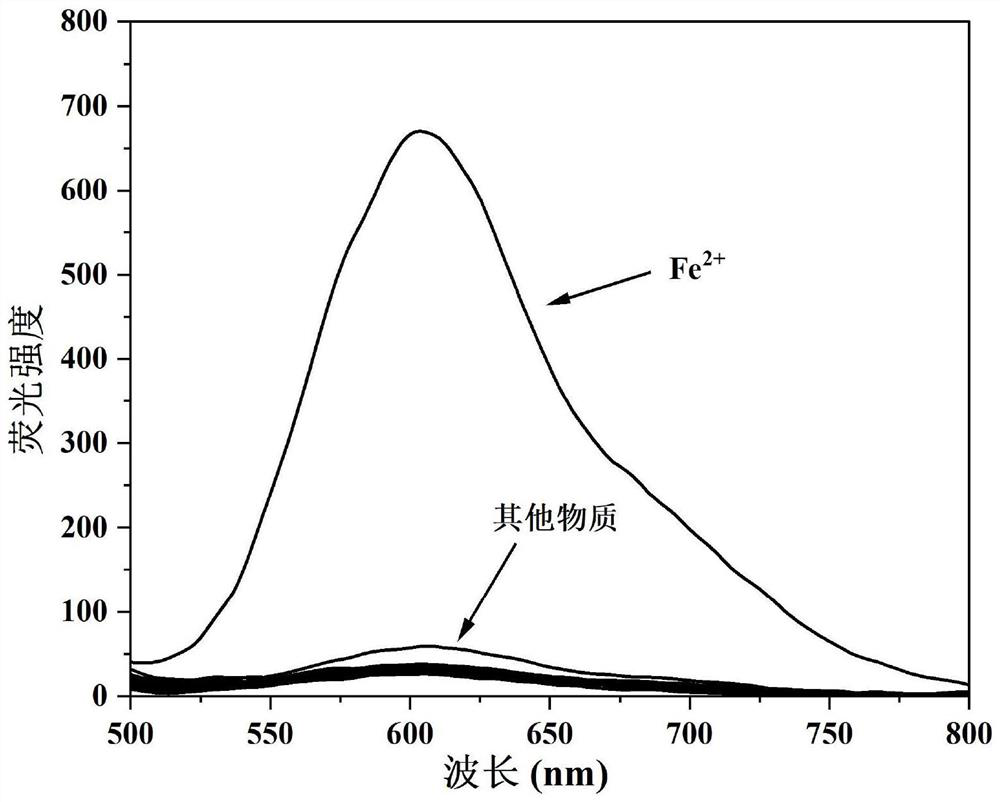
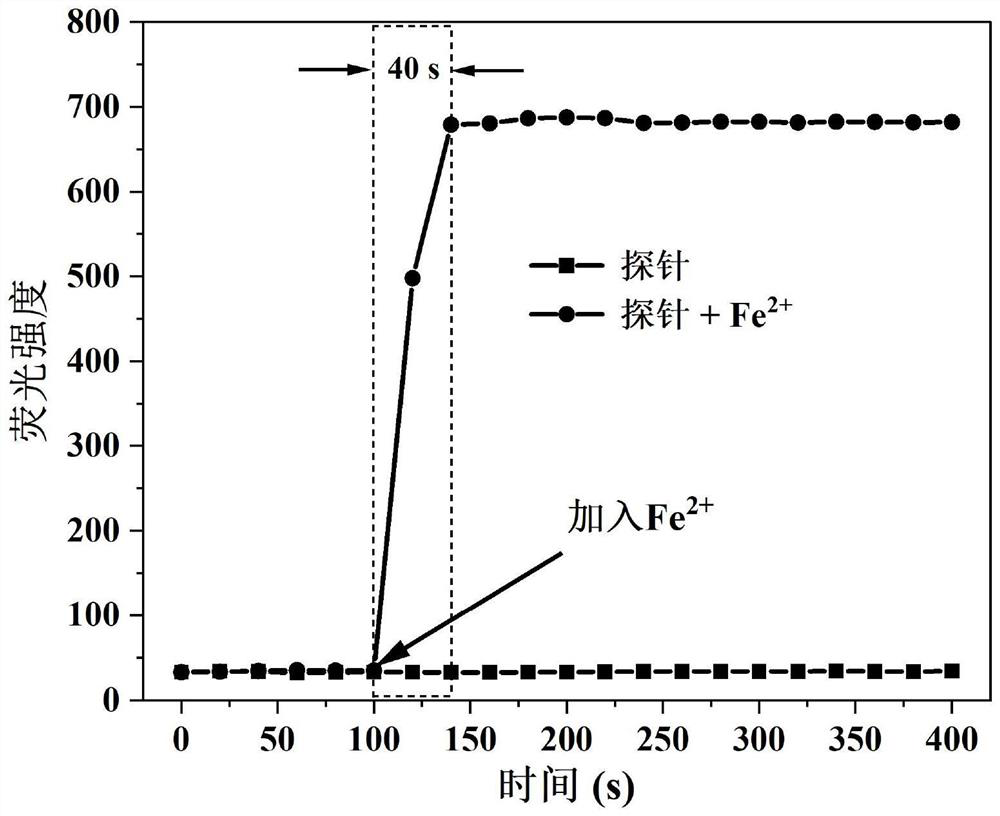
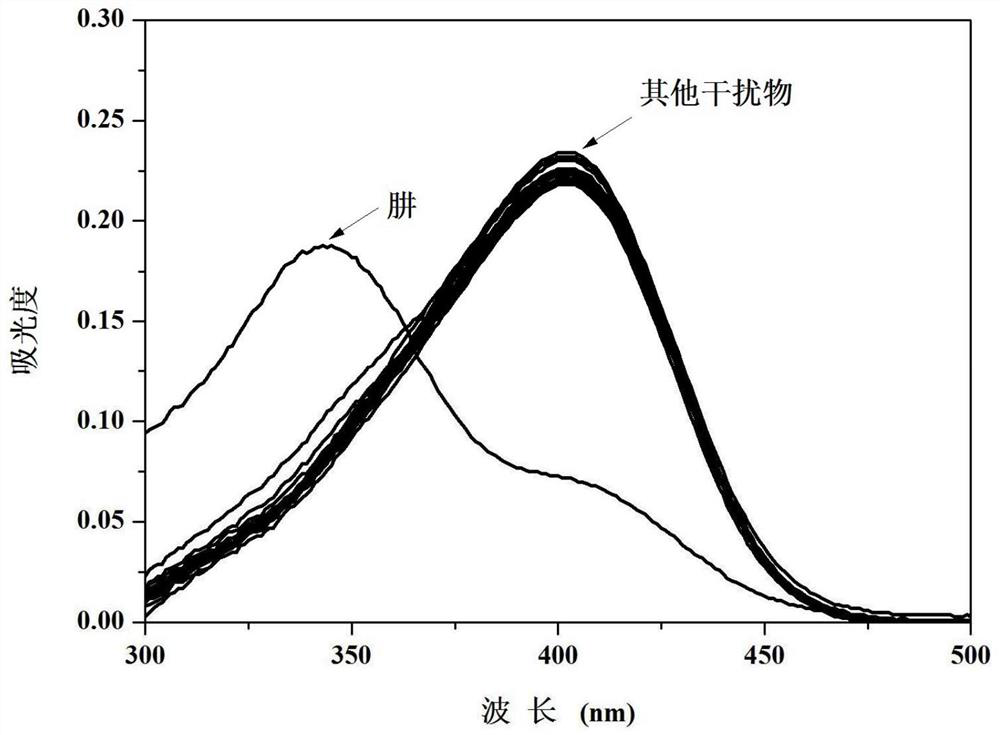
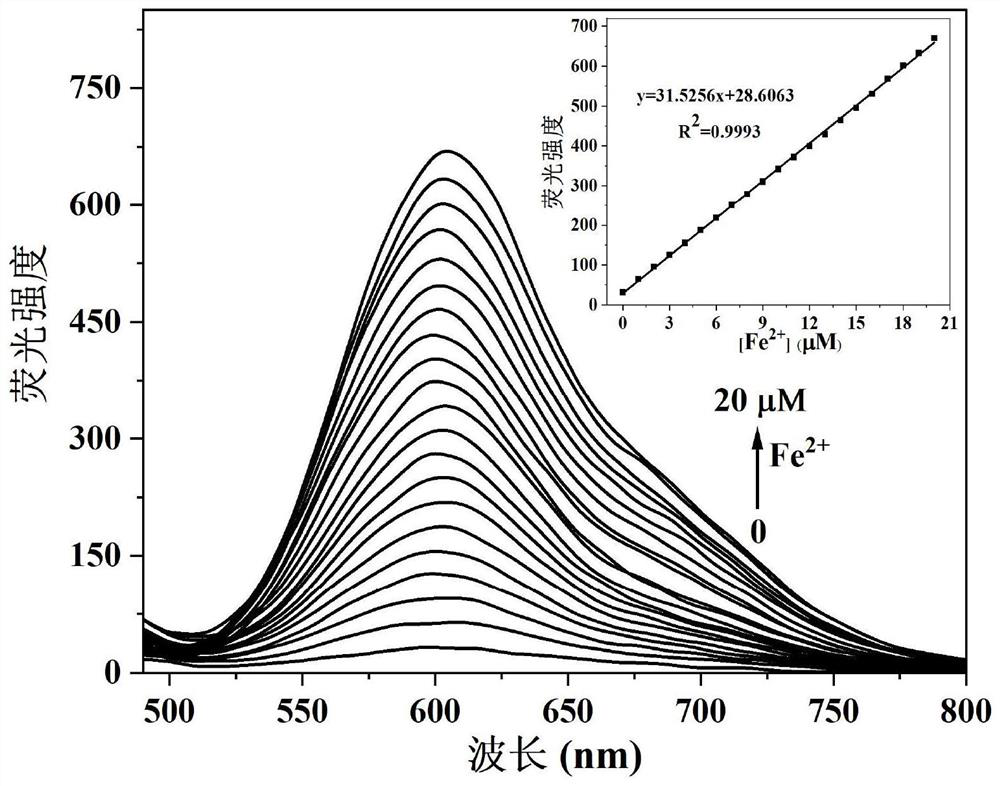
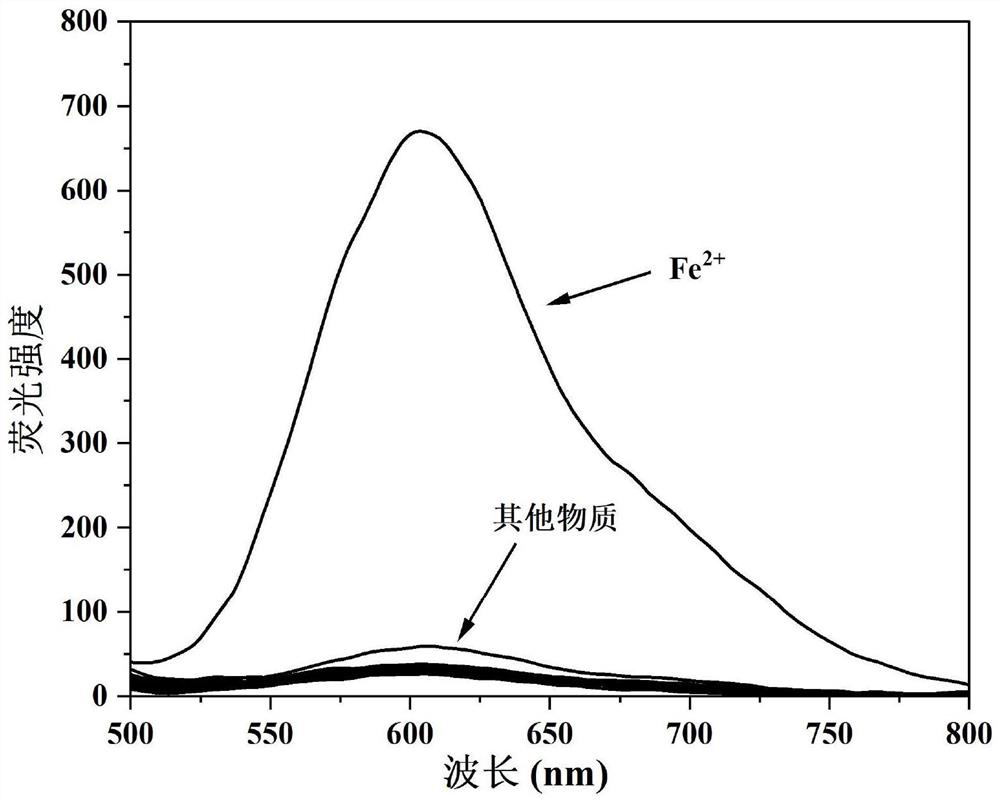
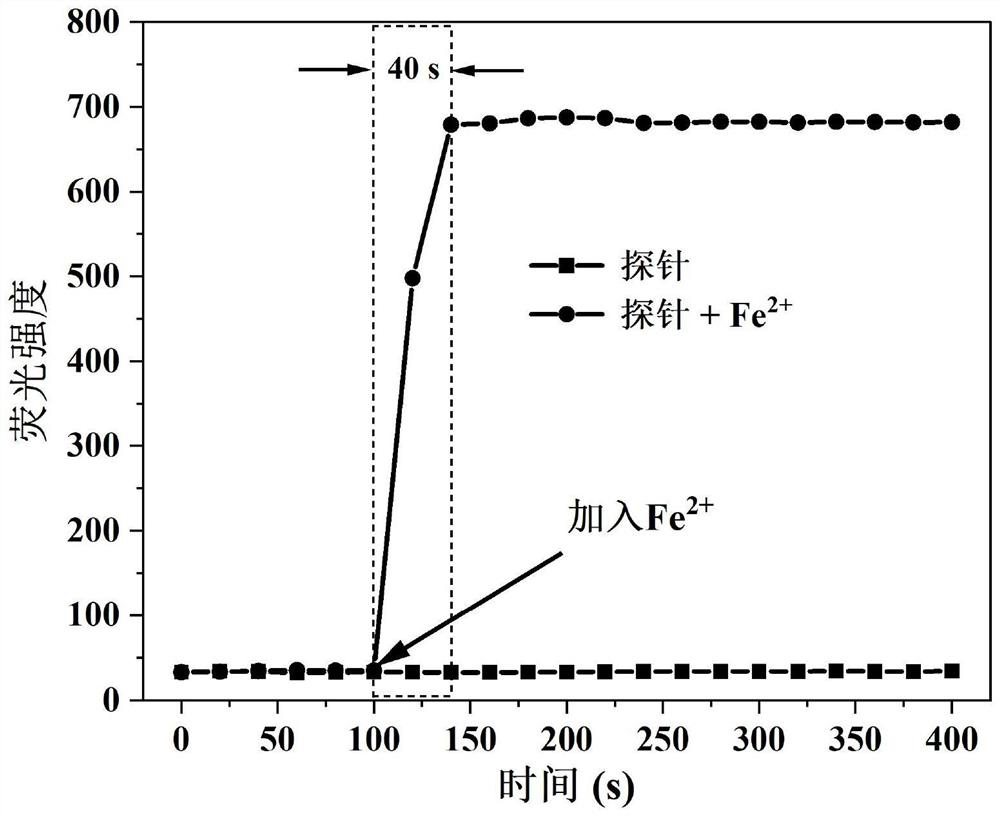
Camphor-based enhanced fluorescent probe for detecting Fe2+ as well as preparation method and application of camphor-based enhanced fluorescent probe
ActiveCN112608255AWide variety of sourcesEasy to synthesizeOrganic chemistryFluorescence/phosphorescenceFluoProbesBenzoic acid
The invention discloses a camphor-based enhanced fluorescent probe for detecting Fe2+ as well as a preparation method and application of the camphor-based enhanced fluorescent probe. The fluorescent probe is N,N-Dimethyl-4-(3-(4,7,7-trimethyl-3-oxybicyclo[2.2.1]hept-2-enyl)prop-1-en-1-yl)aniline oxide. The preparation method comprises the following steps: taking camphor as a raw material, carrying out condensation reaction on camphor and 4-dimethylamino cinnamyl aldehyde to obtain 3-(3-(4-(dimethylamino)phenyl)allylidene)-1,7,7-trimethylbicyclo[2.1.1]heptan-2-one. carrying out oxidation reaction on 3-(3-(4-(dimethylamino)phenyl)allylidene)-1,7,7-trimethylbicyclo[2.1.1]heptan-2-one and m-chloroperoxybenzoic acid to obtain N,N-Dimethyl-4-(3-(4,7,7-trimethyl-3-oxybicyclo[2.2.1]hept-2-enyl)prop-1-en-1-yl)aniline oxide. The compound can specifically recognize Fe2+, and solution fluorescence is changed from colorless to red under 365nm ultraviolet light, so that the compound can be used as an enhanced fluorescent probe for detecting Fe2+, the detection limit reaches 8.3 nM, the response time is within 40s, and the compound has a good application prospect.
Owner:NANJING FORESTRY UNIV
Method for preparing cycloheptanone from cyclohexanone through one step
InactiveCN106380385AShort synthetic routeEasy to operateOrganic compound preparationCarbonyl compound preparationWater vaporDistillation
Owner:HUNAN KEREY BIOTECH
Method for preparing hinokitiol
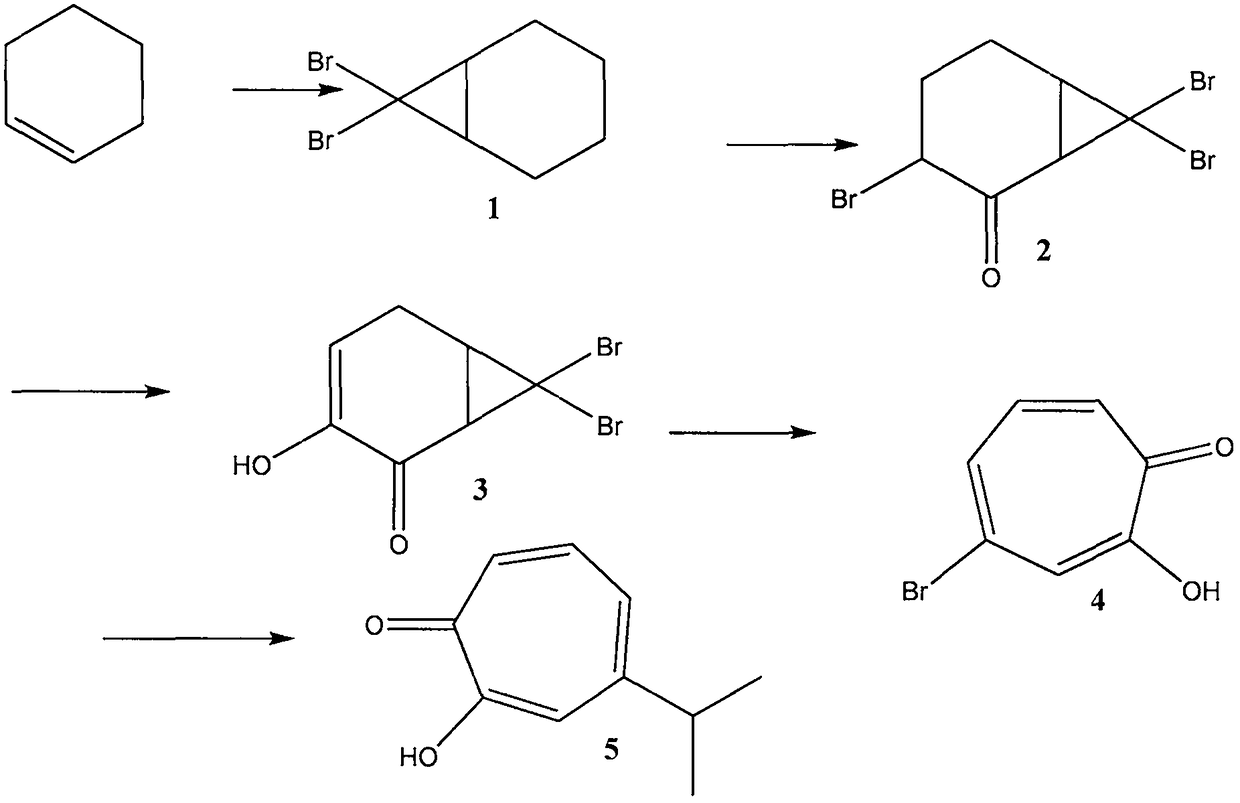
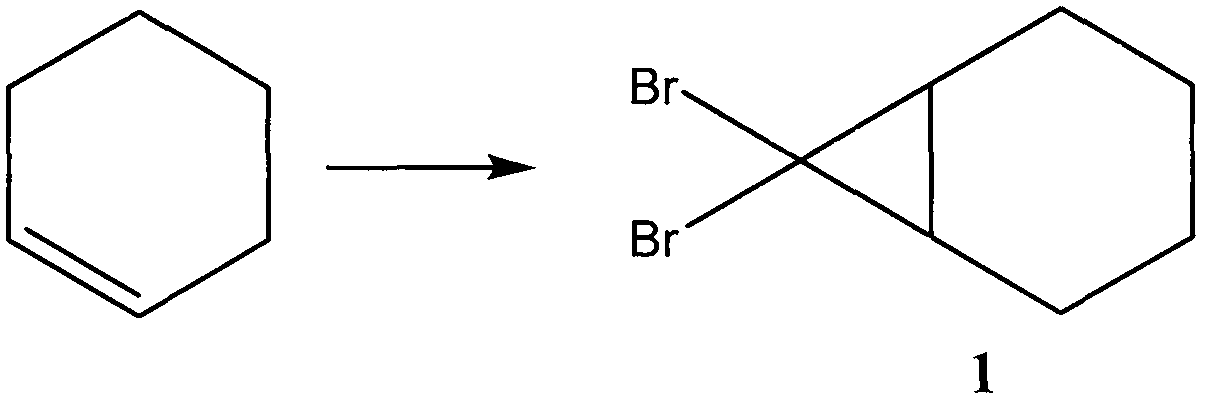
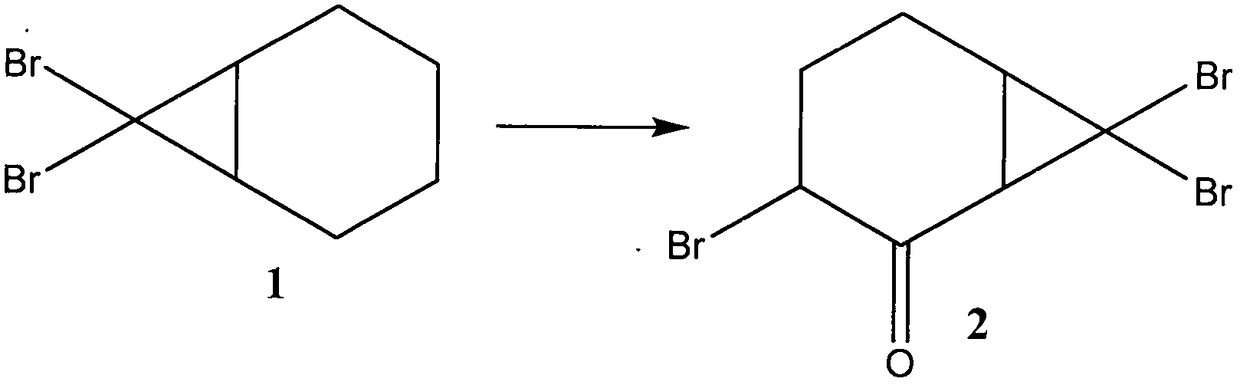
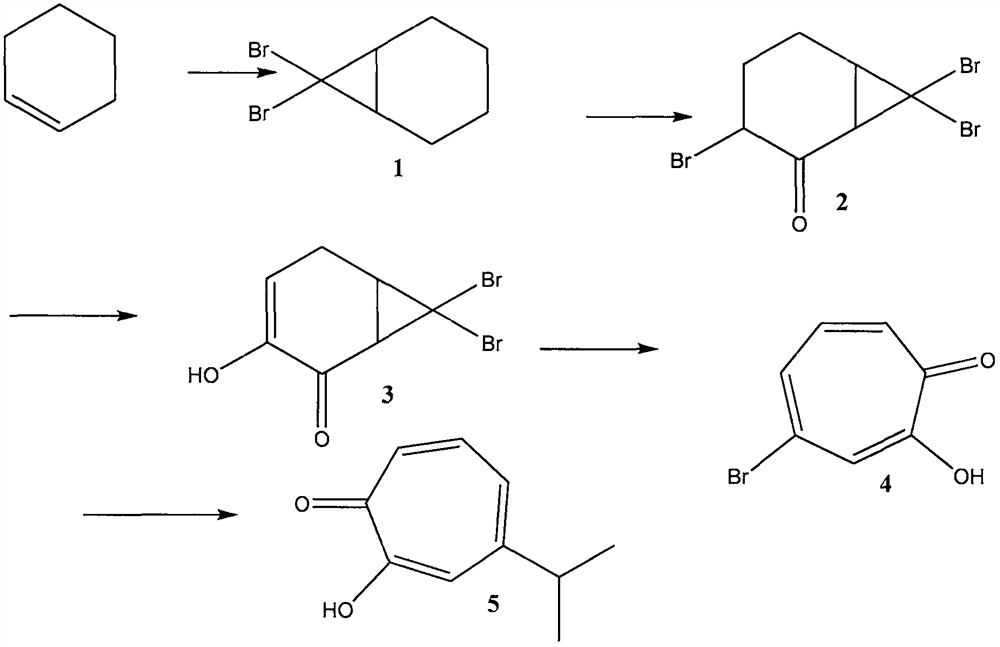
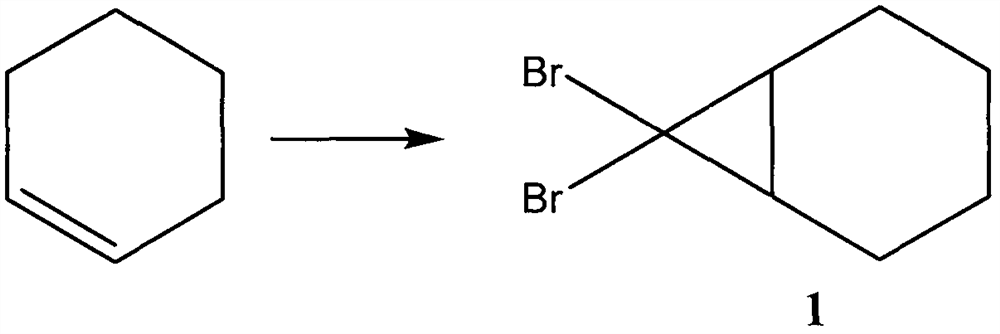
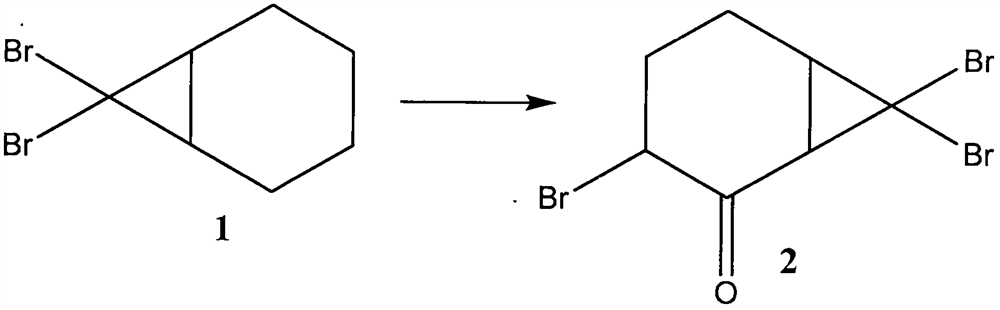
ActiveCN109134229AEliminate potential safety hazardsFix security issuesOrganic compound preparationCarbonyl compound preparation by oxidationAlkyl transferCyclohexene
The invention discloses a method for preparing hinokitiol. The method includes the steps of conducting an addition reaction on bromoform and an initial raw material compound, namely cyclohexene, to obtain a first compound, namely 7,7-dibromo bicyclo[4.1.0]heptane; the first compound is subjected to an oxidization reaction to obtain a second compound, namely 3,7,7-tribromo bicyclo[4.1.0]heptyl-2-ketone; the second compound is subjected to a substitution reaction to obtain a third compound, namely 7,7-dibromo-3-hydroxy bicyclo[4.1.0] heptyl-3-alkene-2-ketone; the third compound is subjected to aring enlargement reaction to obtain a fourth compound, namely 4-bromo-2-hydroxy cycloheptyl-2,4,6-triene-1-ketone; the fourth compound is subjected to an alkylation reaction to obtain hinokitiol.
Owner:INST OF ENVIRONMENT & SUSTAINABLE DEV IN AGRI CHINESE ACADEMY OF AGRI SCI +3
Chiral N-heterocyclic carbene catalyst as well as preparation method and application thereof
ActiveCN113336721AEasy to operateHigh yieldOrganic compound preparationOrganic chemistry methodsMethylanilinePtru catalyst
Owner:CHENGDU UNIV
6,7-dihydro-5H-diphenyl[a,c]cyclohepta-5-ketone compound and preparation method thereof
InactiveCN104230689AEasy to operateWide range of substratesCarboxylic acid nitrile preparationOrganic compound preparationArylOrganic solvent
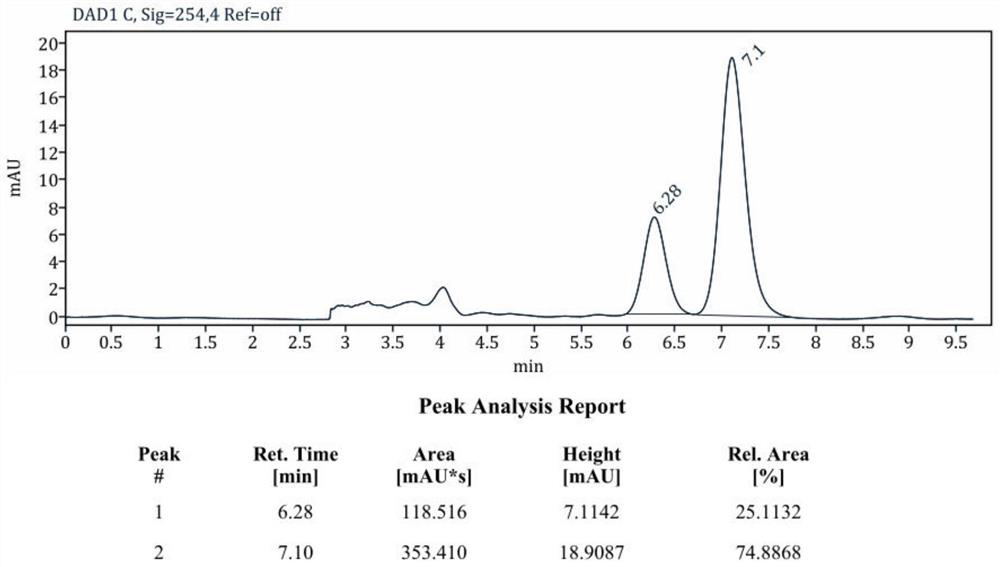
The invention relates to a 6,7-dihydro-5H-diphenyl[a,c]cyclohepta-5-ketone compound and a preparation method thereof. A formula of the compound is as shown in the specification. O-bromoaryl methanol, aryl ethanone, [Ir(cod)Cl]2, a palladium salt, a sliver salt, 1,1'-binaphthyl-2,2'-bisdiphenylphosphine and alkaline are taken and added into an organic solvent, heated and refluxed in the presence of an N2 gas, filtered, evaporated and re-crystallized after the reaction is ended to obtain the 6,7-dihydro-5H-diphenyl[a,c]cyclohepta-5-ketone compound. The 6,7-dihydro-5H-diphenyl[a,c]cyclohepta-5-ketone compound is synthesized by one step, wherein the reaction substrate scope is wide, the yield is high, the reaction is economical and efficient, and the application prospect is wide.
Owner:LUOYANG NORMAL UNIV
A camphor-based fluorescent probe for detecting hydrazine and its preparation method
InactiveCN109232444BStrong blue fluorescenceGood practical valueOrganic chemistryFluorescence/phosphorescenceFluoProbesFluorescence
The invention discloses a camphor-based fluorescent probe for detecting hydrazine and a preparation method thereof. The camphor-based fluorescent probe is: 4-(4′-dimethylaminophenyl)-8,9,9-trimethyl-5,6,7,8-tetrahydro-5,8-endomethylene Quinazoline-2-amine. The present invention utilizes the natural renewable resource camphor as a raw material to condense with p-dimethylaminobenzaldehyde to generate 3-(4'-dimethylaminobenzylidene)-1,7,7-trimethylbicyclo[2.1.1 ]heptane-2-one; 3-(4′-dimethylaminobenzylidene)-1,7,7-trimethylbicyclo[2.1.1]heptane-2-one and then carry out condensation cyclization with guanidine hydrochloride , to obtain 4-(4'-dimethylaminophenyl)-8,9,9-trimethyl-5,6,7,8-tetrahydro-5,8-methanoquinazoline-2- amine. The compound can specifically react with hydrazine to emit significantly enhanced blue fluorescence, and can be used as a fluorescent probe for the detection of hydrazine, and has good practical value.
Owner:NANJING FORESTRY UNIV
One for detection of fe 2+ Camphor-based enhanced fluorescent probe and its preparation method and application
ActiveCN112608255BWide variety of sourcesEasy to synthesizeOrganic chemistryFluorescence/phosphorescenceFluoProbesBenzoic acid
The invention discloses a method for detecting Fe 2+ The camphor-based enhanced fluorescent probe and its preparation method and application. The fluorescent probe is N,N-dimethyl-4-(3-(4,7,7-trimethyl-3-oxybicyclo[2.2.1]hept-2-enyl)prop-1-ene ‑1‑yl) aniline oxide. The present invention utilizes camphor as raw material, carries out condensation reaction with 4-dimethylaminocinnamaldehyde, obtains 3-(3-(4-(dimethylamino)phenyl) allylidene)-1,7,7-trimethyl 3-(3-(4-(dimethylamino)phenyl)allylidene)-1,7,7-trimethylbicyclo[2.1.1] Heptyl-2-one is oxidized with m-chloroperoxybenzoic acid to obtain N,N-dimethyl-4-(3-(4,7,7-trimethyl-3-oxybicyclo[2.2.1] Hep-2-enyl)prop-1-en-1-yl)aniline oxide. The compound can specifically recognize Fe 2+ , under 365nm ultraviolet light, the fluorescence of the solution changes from colorless to red, so it can be used to detect Fe 2+ The enhanced fluorescent probe has a detection limit of 8.3nM and a response time of 40s, which has a good application prospect.
Owner:NANJING FORESTRY UNIV
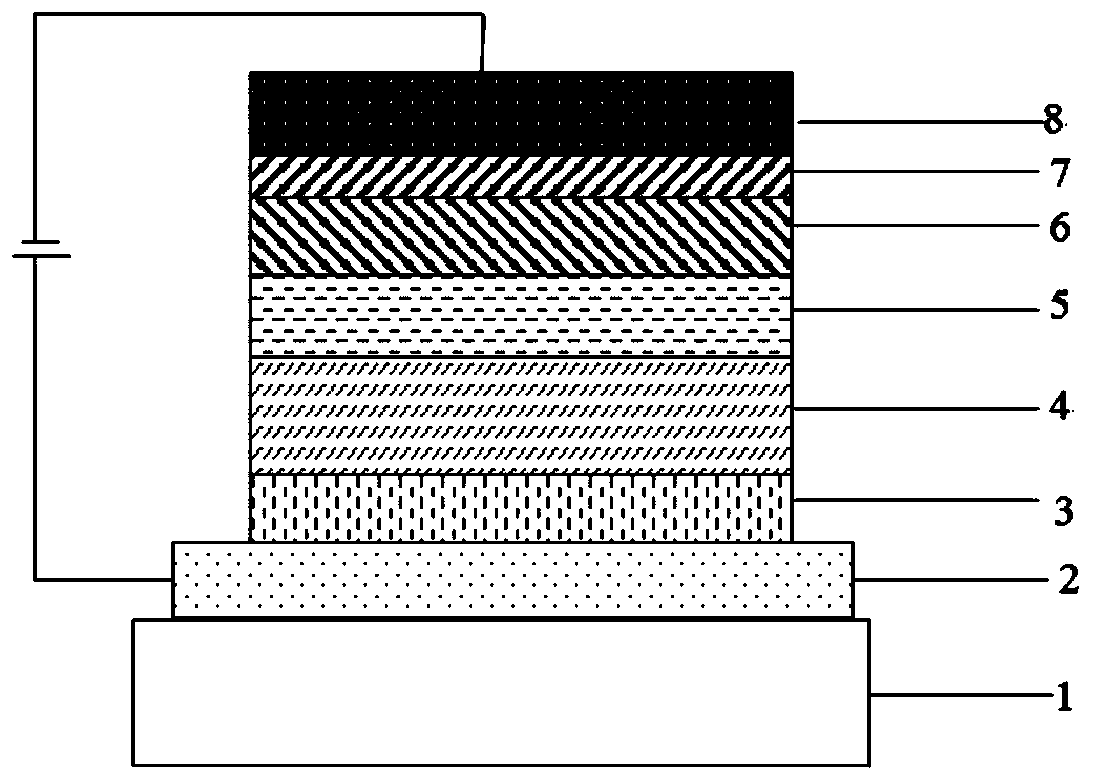
A compound with azadibenzocycloheptanone as the core and its application in oled
ActiveCN107021925BBreak symmetryDestroy crystallinityOrganic chemistrySolid-state devicesQuantum efficiencyCycloheptanone
The invention discloses a compound with azadibenzocycloheptanone as the core and its application in organic electroluminescent devices. This type of compound takes azadibenzocycloheptanone as the core, and the material is not easy to crystallize, and has the characteristics of good film formation and thermal stability; at the same time, it has a smaller triplet state than dibenzocycloheptanone. It is different from the singlet state energy, and can make full use of the triplet state energy. When the compound of the present invention is used as a light-emitting layer material of an OLED light-emitting device, the current efficiency, power efficiency and external quantum efficiency of the device are greatly improved; at the same time, the life of the device is significantly improved.
Owner:JIANGSU SUNERA TECH CO LTD
The synthetic method of harringtonine C ring intermediate
The invention relates to an artificial synthetic method of a harringtonine C-ring intermediate. According to the method, 3,4-methylenedioxyphenethylamine (compound I) as a raw material is subjected to acylation in alkaline conditions to obtain N-acylated-3,4-methylenedioxyphenethylamine (compound II), which is then reacts with halogenated acetic acid or halogenated acetic acid derivative to generate N-acylated-3,4-methylenedioxyphenethylamine acetic acid (compound III); and the compound (III) is catalyzed by Lewis acid to generate intramolecular Friedel-crafts acylation reaction to obtain N-acyl-3,4-metheneoxybenzo-3-N-cycloheptanone, namely C ring of harringtonine. In the preparation process, products of each step are purified by washing and recrystallization; and the method has simple operation, total yield of 87.4%, and good industrial prospect.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Compound for inhibiting the growth and proliferation of human liver cancer cells and method for synthesizing it
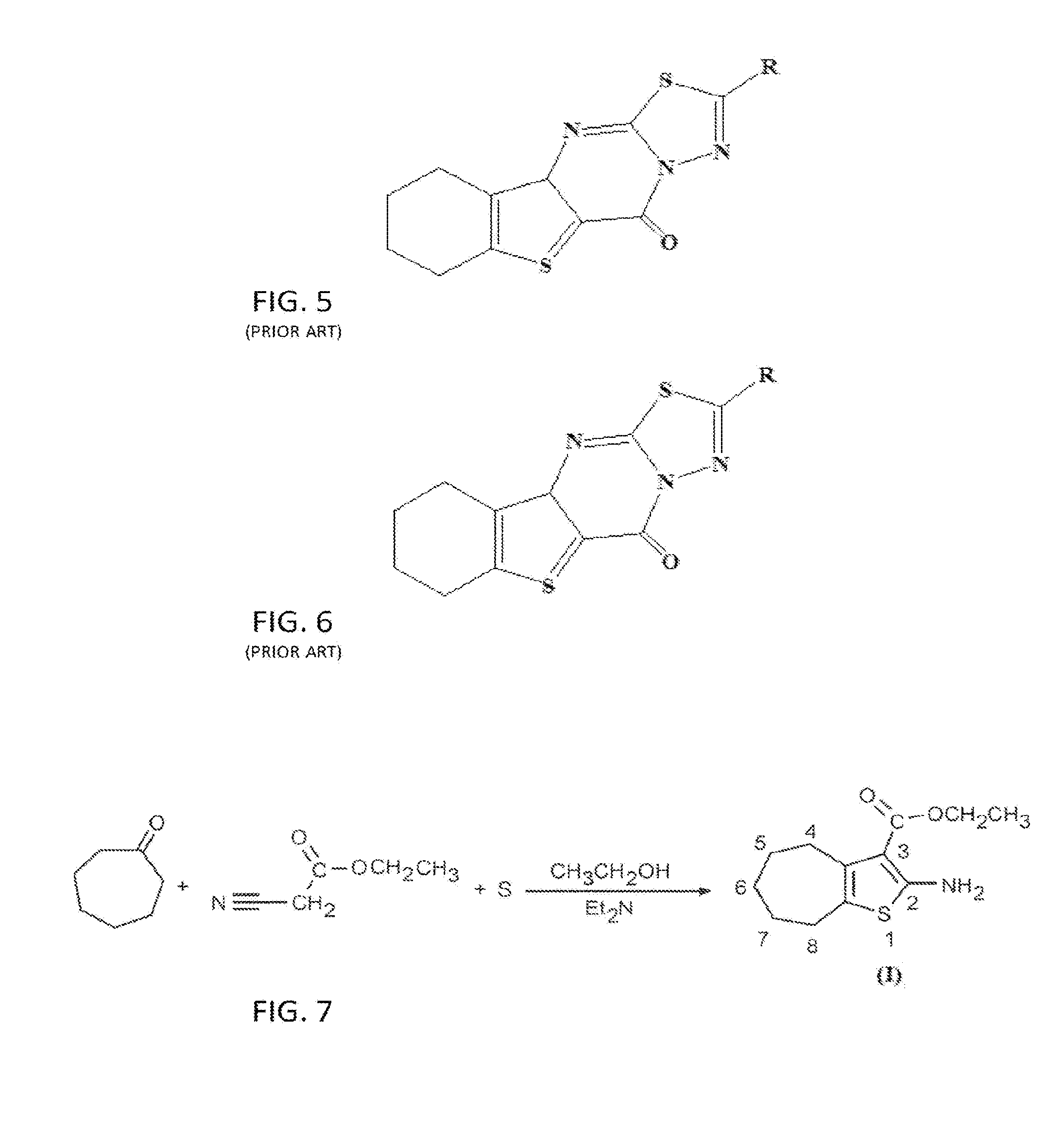
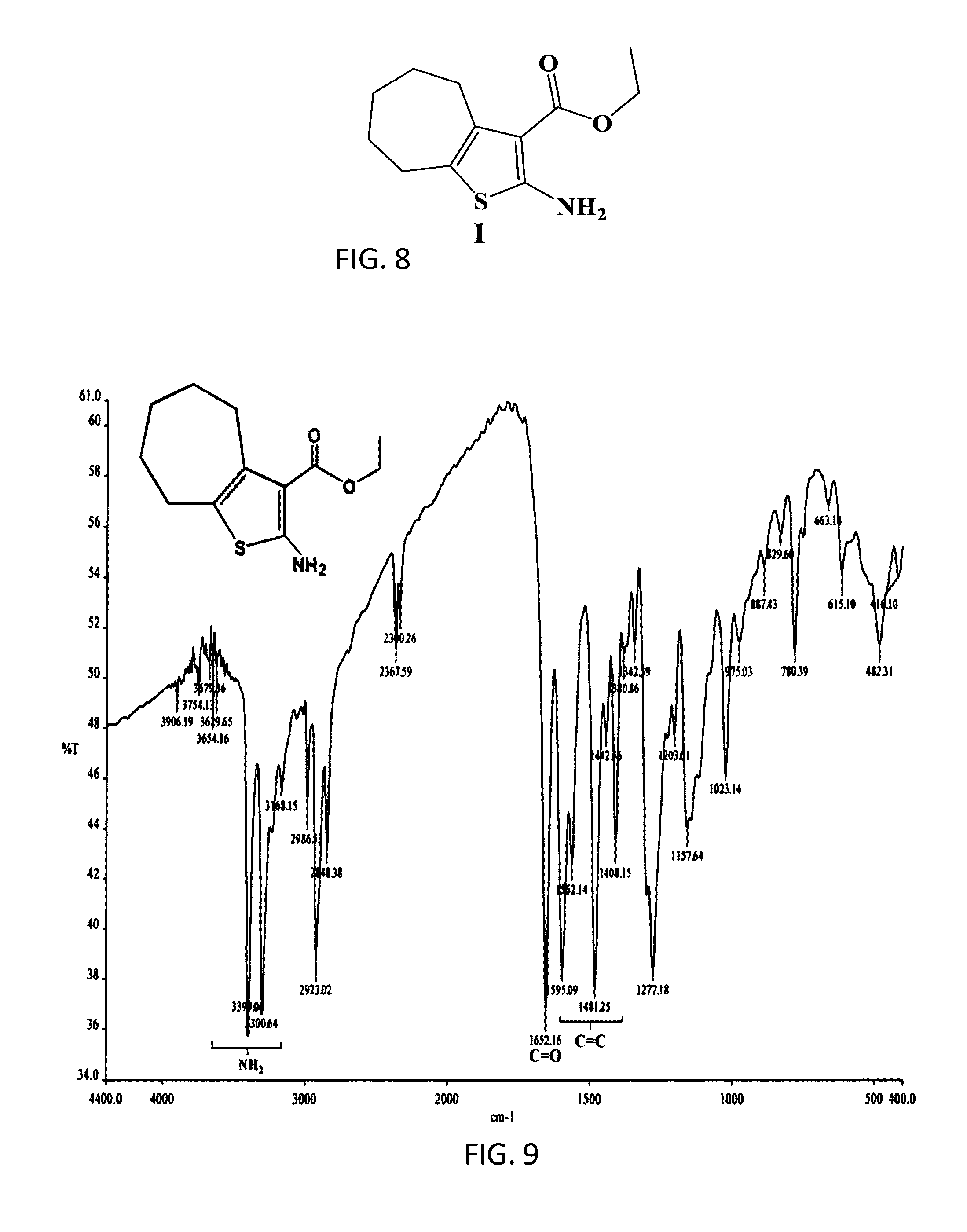
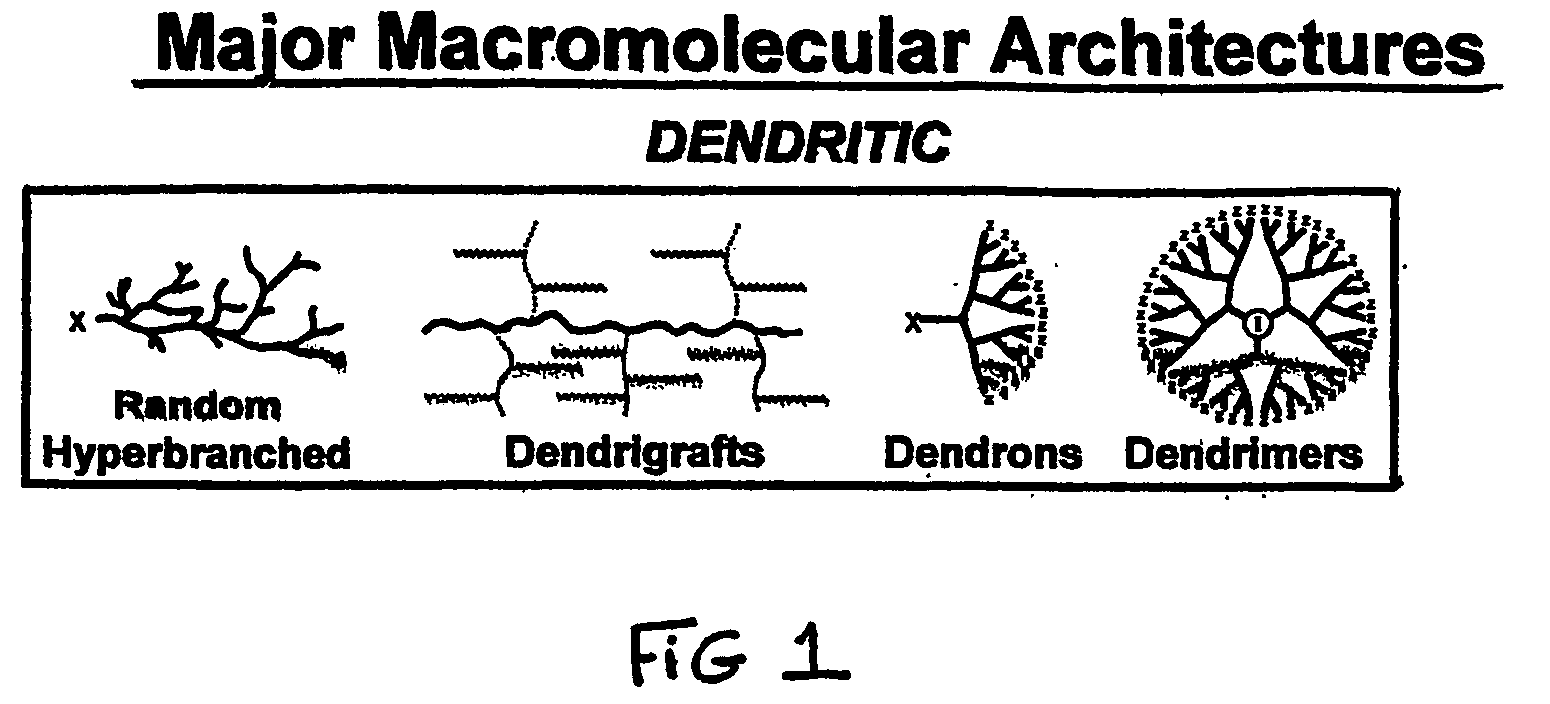
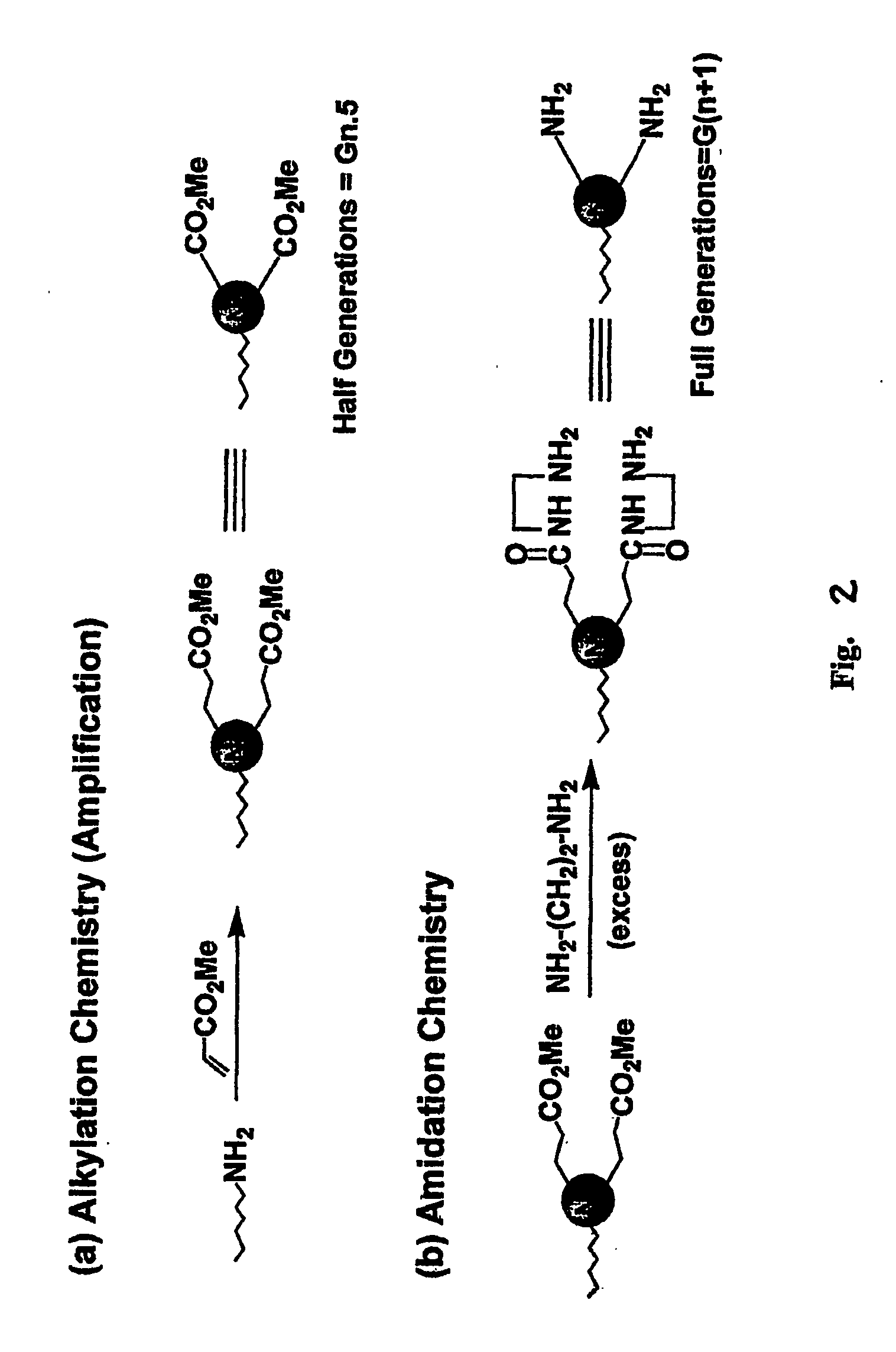
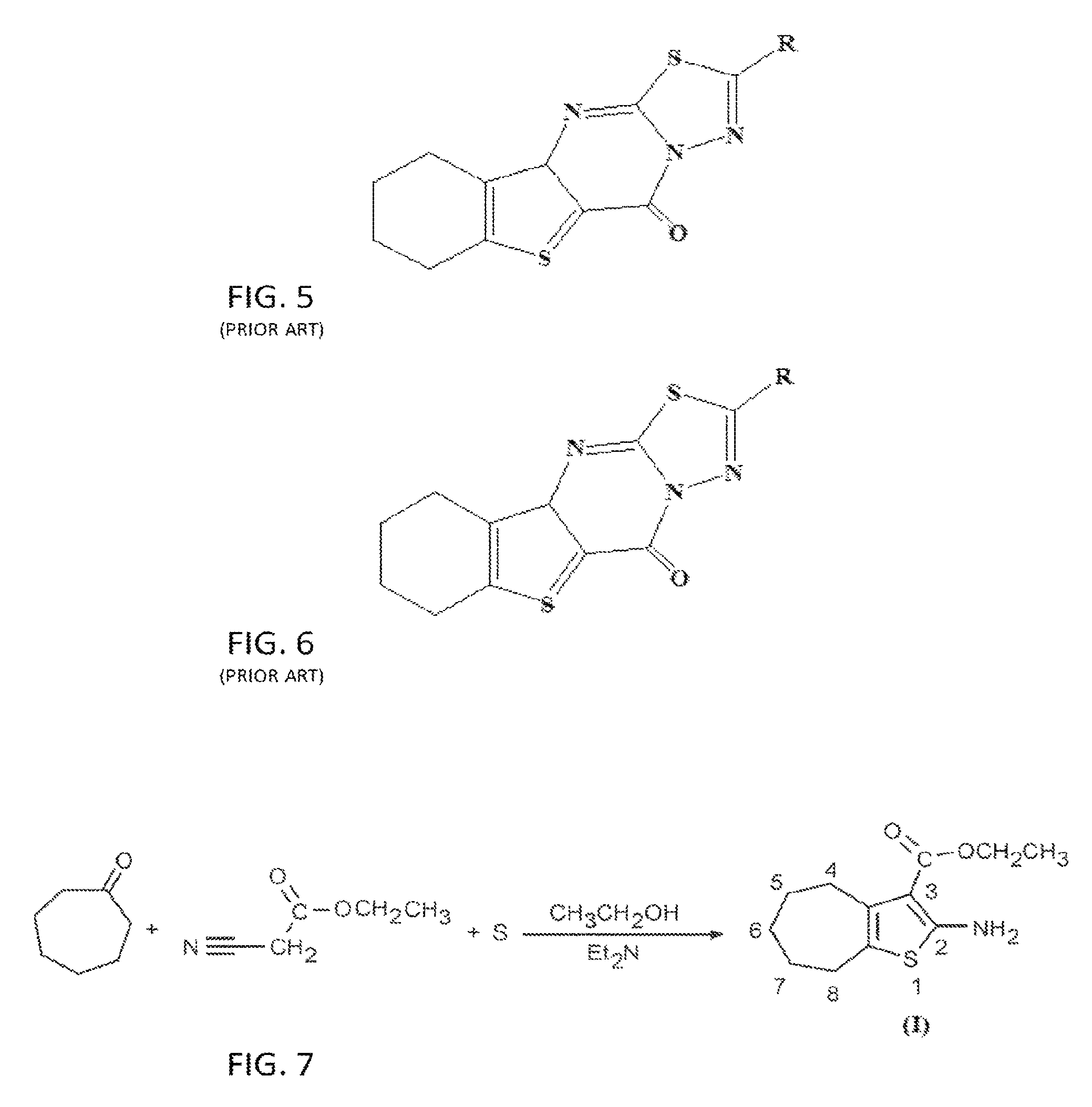
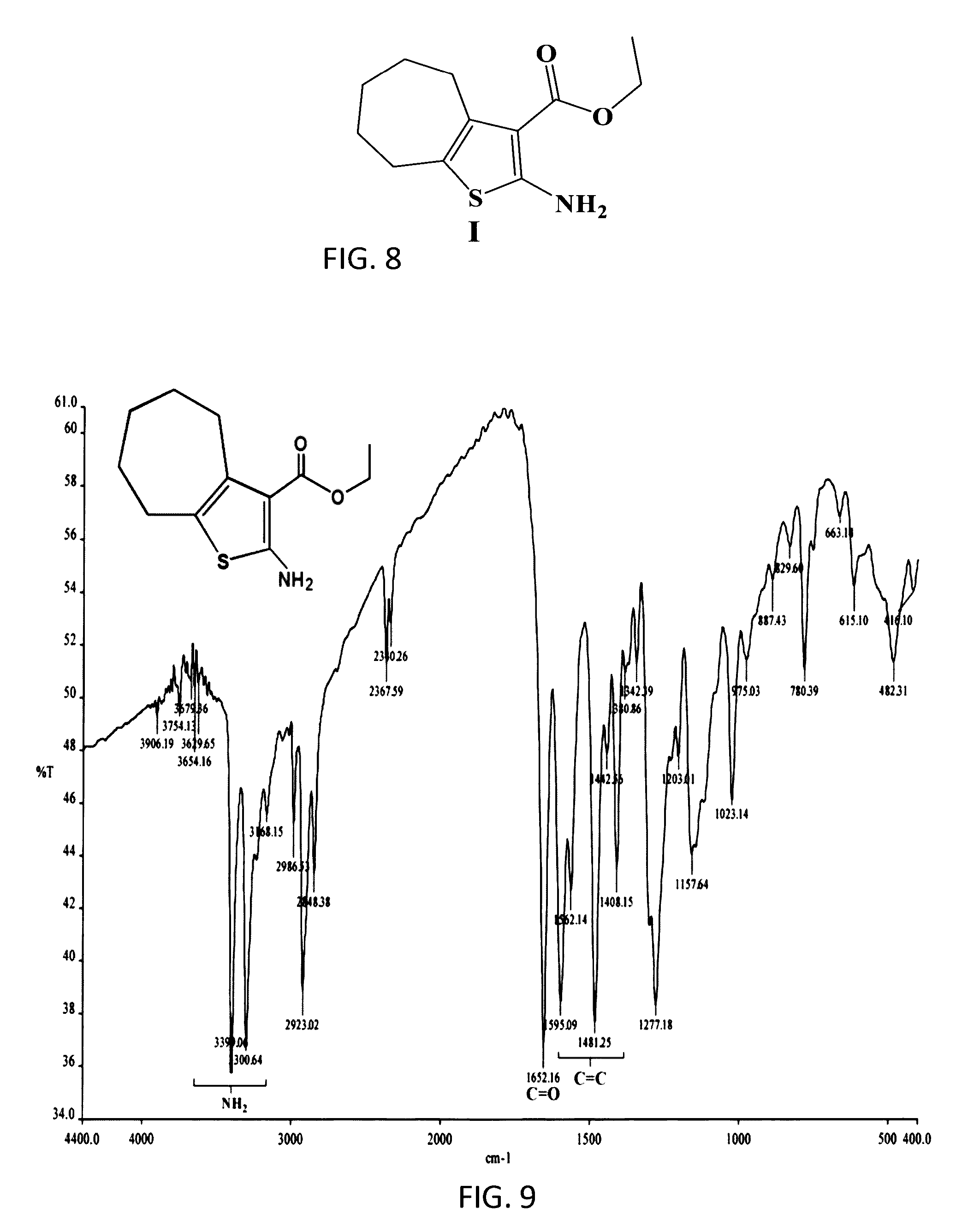
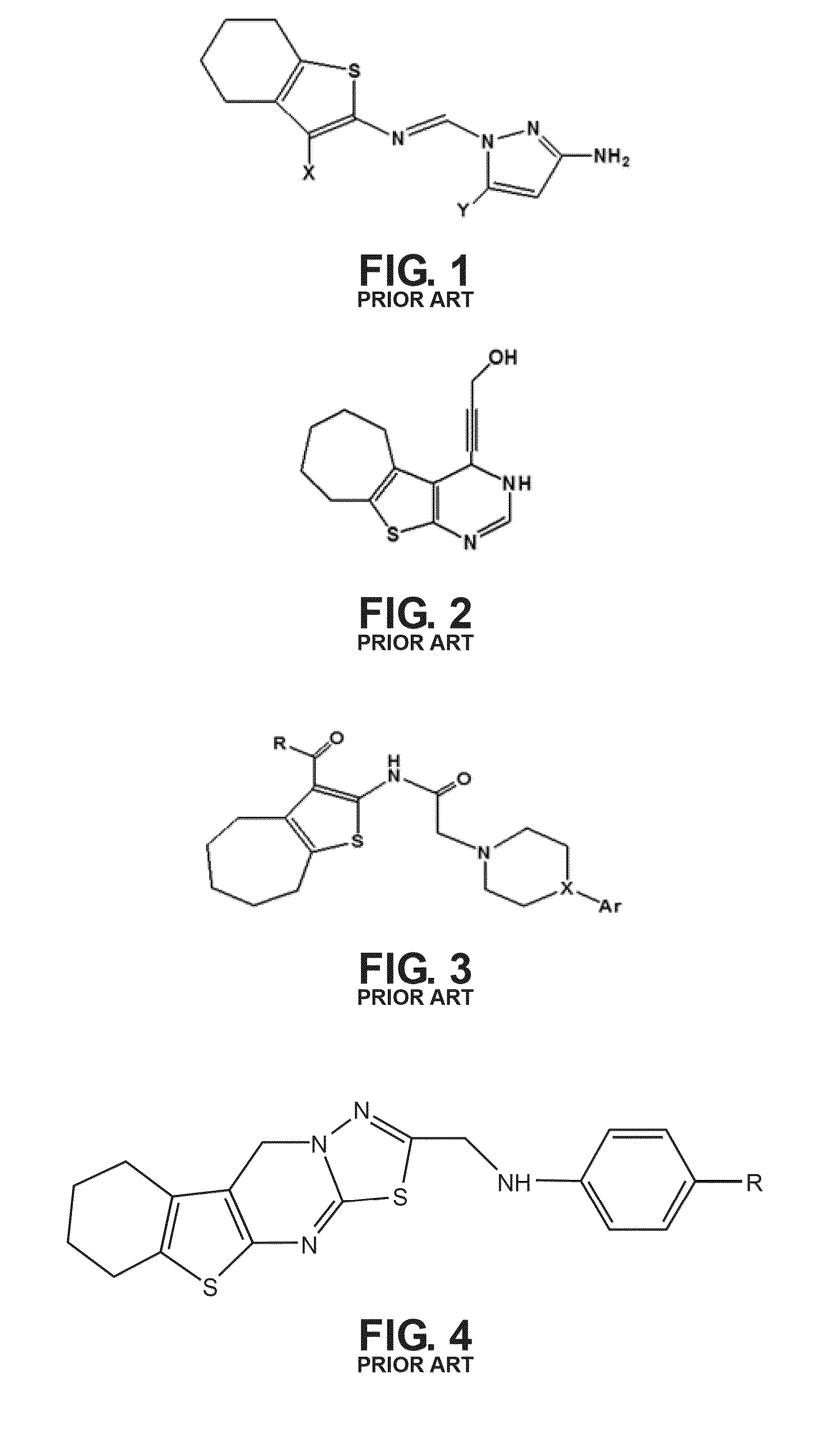
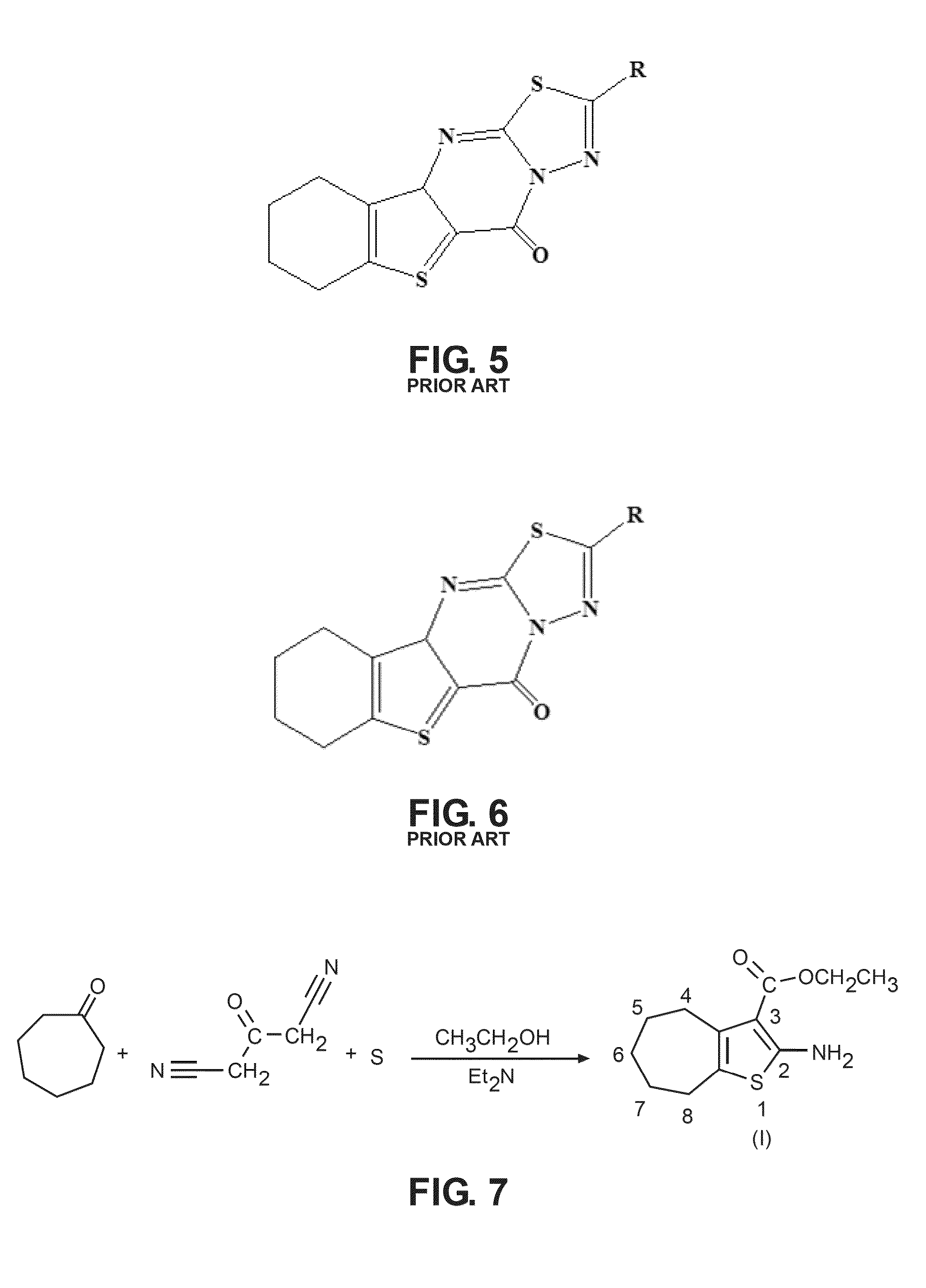
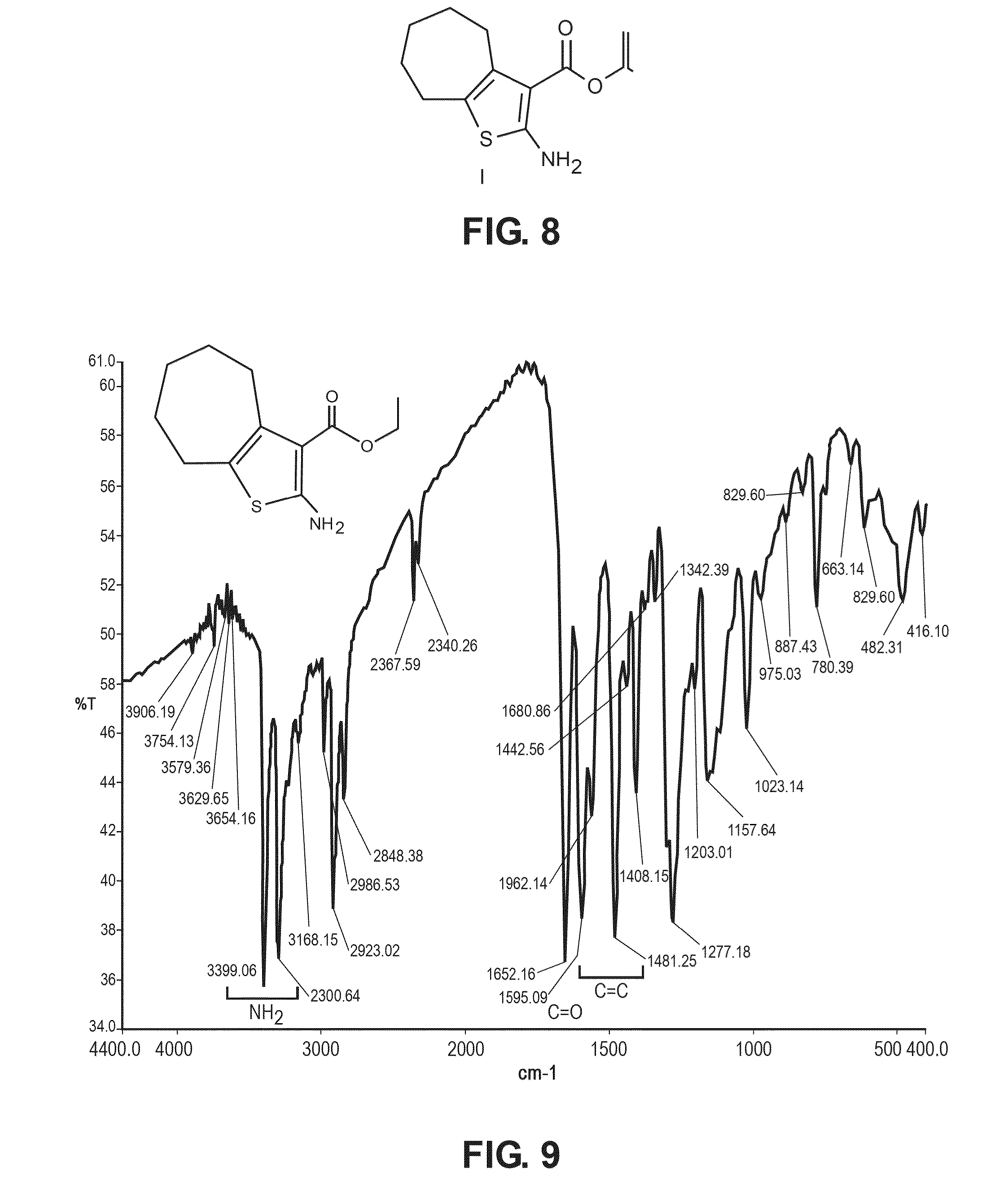
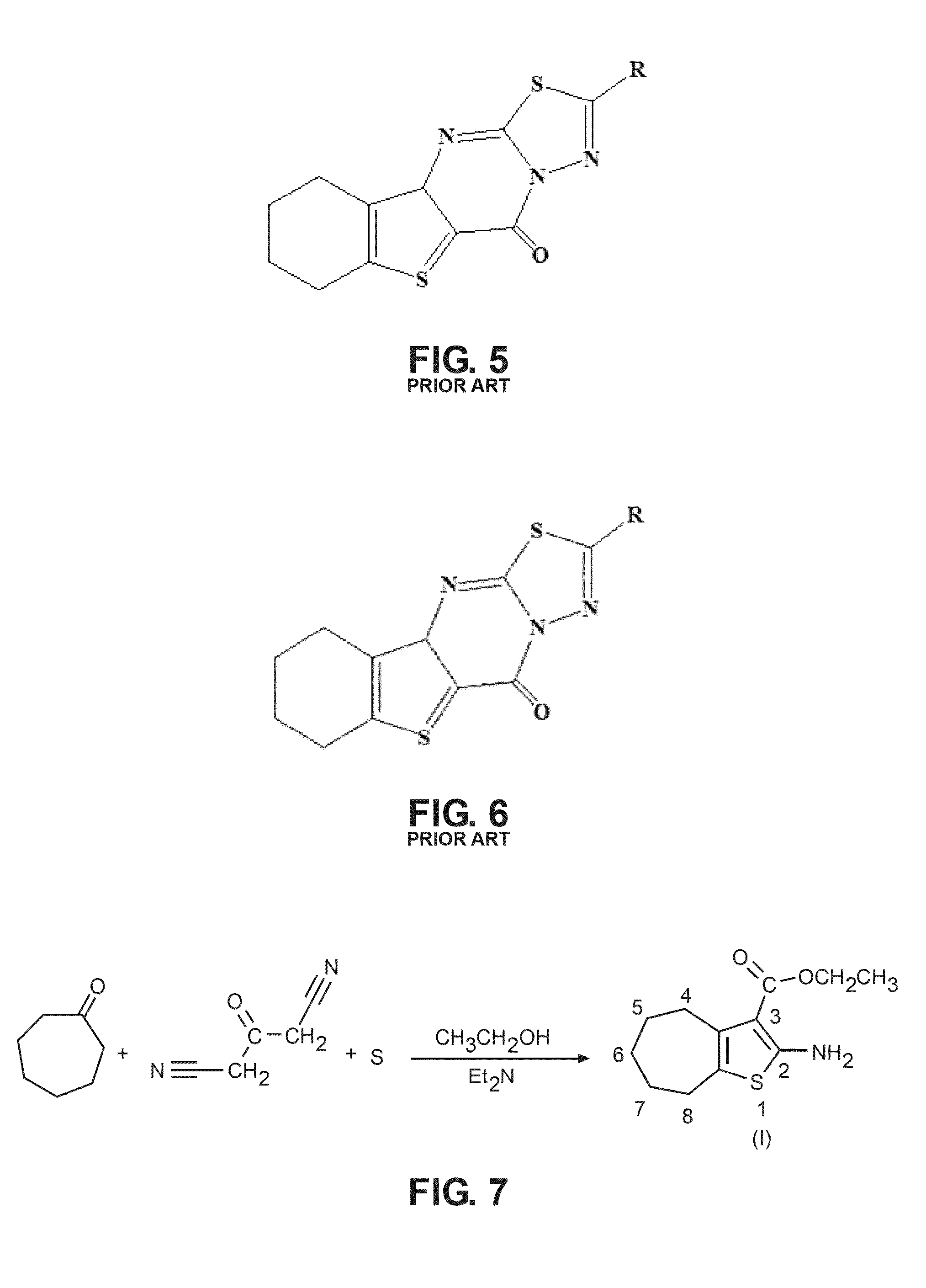
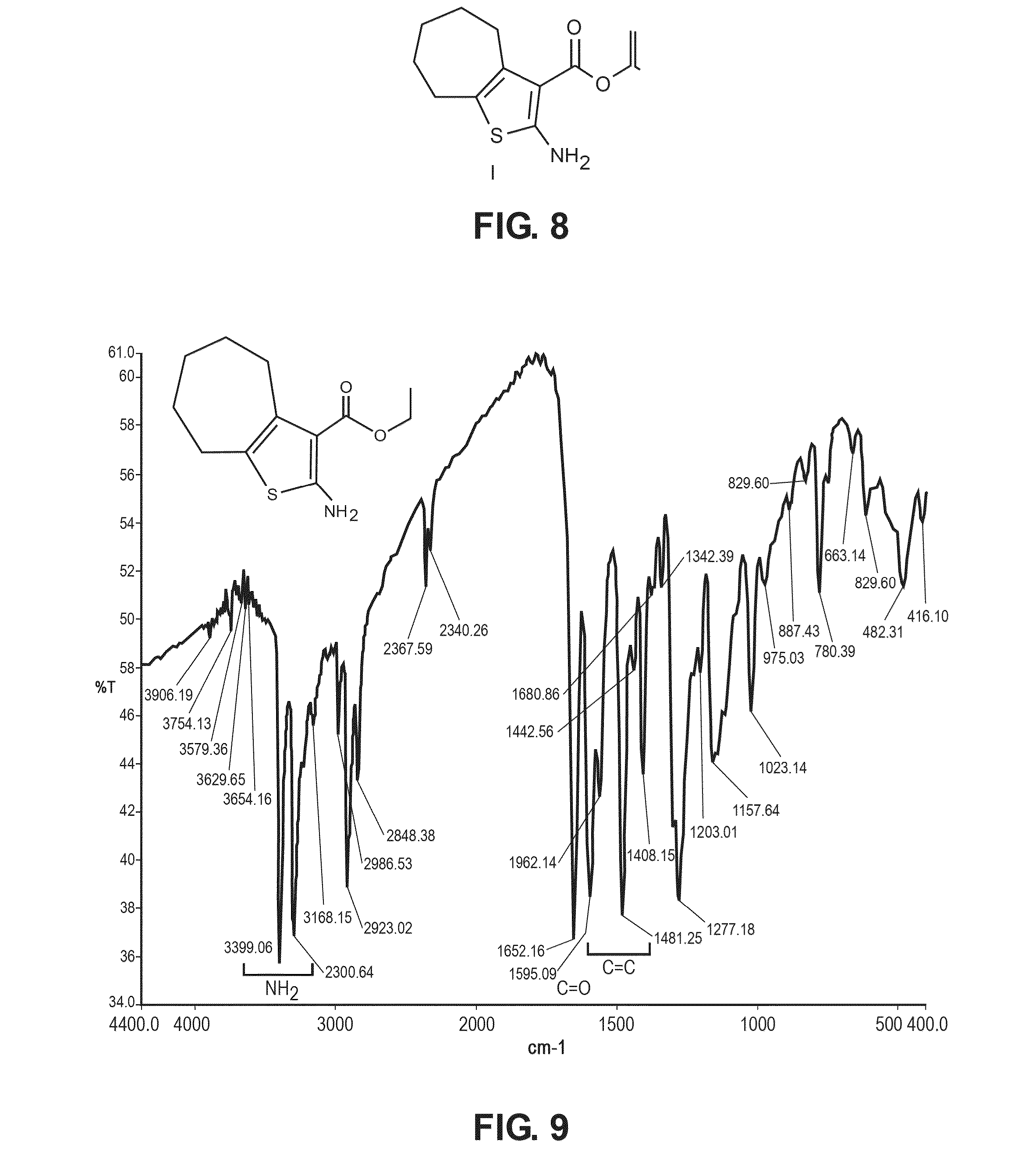
The compound “2-((4-nitrophenyl)amino)-6,7,8,9-tetrahydro-3H-cyclohepta[4,5]thieno-[2,3-d]pyrimidin-4(5H)-one” and method of synthesizing it, wherein the compound is effective to inhibit the growth and proliferation of human liver cancer cells HepG2. The compound has an inhibitory concentration value (IC50) of 0.7 μg, compared to reference medication Doxorubicin that has an (IC50) value of 1.2 μg. It further surpasses that reference medication Doxorubicin at all tested concentraions. The method includes the steps of: preparing a first compound of cycloheptanone, ethylcyanoacetate, sulfur, ethanol and diethyl amine; preparing a second compound by heating of the first compound with excess of hydrazine hydrate in absolute ethanol as solvent; and preparing the effective compound of the invention by reaction of the second chemical compound with 4-nitrophenylisothiocyanate in dry dimethylformamide as solvent.
Owner:ALGHAMDI ZAINAB SAEED
A kind of method for preparing hinoki alcohol
ActiveCN109134229BEliminate potential safety hazardsFix security issuesOrganic compound preparationCarbonyl compound preparation by oxidationCyclohexeneCycloheptanone
The invention discloses a method for preparing hinokitiol. The method includes the steps of conducting an addition reaction on bromoform and an initial raw material compound, namely cyclohexene, to obtain a first compound, namely 7,7-dibromo bicyclo[4.1.0]heptane; the first compound is subjected to an oxidization reaction to obtain a second compound, namely 3,7,7-tribromo bicyclo[4.1.0]heptyl-2-ketone; the second compound is subjected to a substitution reaction to obtain a third compound, namely 7,7-dibromo-3-hydroxy bicyclo[4.1.0] heptyl-3-alkene-2-ketone; the third compound is subjected to aring enlargement reaction to obtain a fourth compound, namely 4-bromo-2-hydroxy cycloheptyl-2,4,6-triene-1-ketone; the fourth compound is subjected to an alkylation reaction to obtain hinokitiol.
Owner:INST OF ENVIRONMENT & SUSTAINABLE DEV IN AGRI CHINESE ACADEMY OF AGRI SCI +3
Chemical compound for inhibiting the growth and proliferation of human liver cancer cells HepG2 and method for synthesizing it
The compound “2-((4-nitrophenyl)amino)-7,8,9,10-tetrahydro cyclohepta[4,5]thieno[2,3-d][1,3,4]thiadiazolo[3,2-a]pyrimidin-11(6H)-one” and method of synthesizing it, wherein the compound is effective to inhibit the growth and proliferation of human liver cancer cells HepG2. The compound has a higher efficiency to inhibit the growth and proliferation of these cells as it has an inhibitory concentration value (IC50) of 0.7 μg, compared to reference medication Doxorubicin that has an (IC50) value of 1.2 μg. It further surpasses that reference medication at all tested concentrations. The method includes the steps of: preparing a first compound of cycloheptanone, ethylcyanoacetate, sulfur, ethanol and diethylamine; preparing a second compound by heating of the first compound with excess of hydrazine hydrate in absolute ethanol as solvent; preparing a third compound by heating the second compound with carbon disulphide in dry pyridine; and preparing the compound of the invention by reacting the third compound with 4-nitrophenylisothiocyanate in dry N,N-methylformamide as solvent.
Owner:ALGHAMDI ZAINAB SAEED
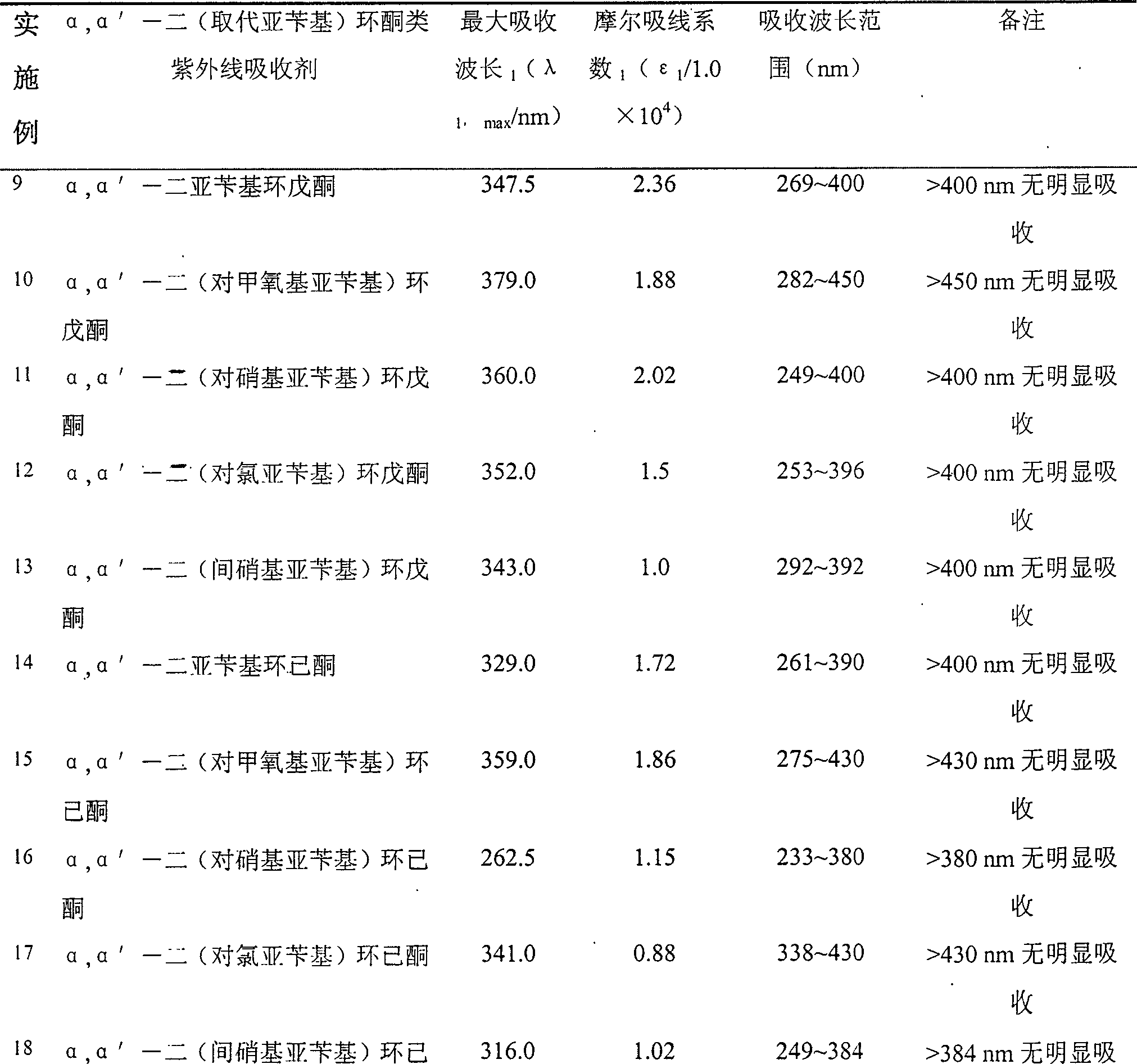
Method of preparing alpha,alpha'-di(substituted benzylidene)cyclone ultraviolet radiation absorbent
InactiveCN101054348AOther chemical processesOrganic compound preparationAcetic acidUltraviolet radiation
The invention relates to a method for preparing a class of ultraviolet absorber of alpha, alpha'-di(substituted benzilidene)ring ketone, in which various alpha, alpha'-di(substituted benzilidene)ring ketone compounds, alpha, alpha'-di(substituted benzilidene)cyclohexanones compounds and alpha, alpha'-di(substituted benzilidene)cycloheptanone compounds are generated through taking mixed solution of concentrated sulfuric acid with acetic as reaction solution and reacting under room temperature condition. The ultraviolet absorber of alpha, alpha'-di(substituted benzilidene)ring ketone compounds has stable chemical properties, can intensely absorb ultraviolet, in which its absorption range is 230-400n, and it can be used as wide spectrum ultraviolet absorbent.
Owner:ZHEJIANG UNIV
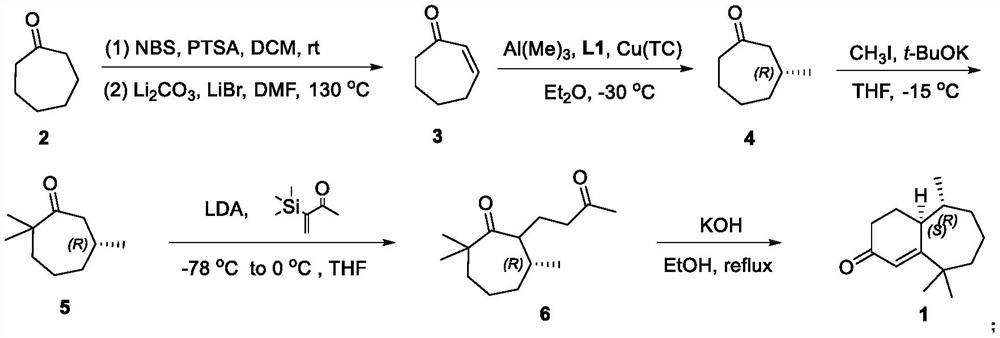
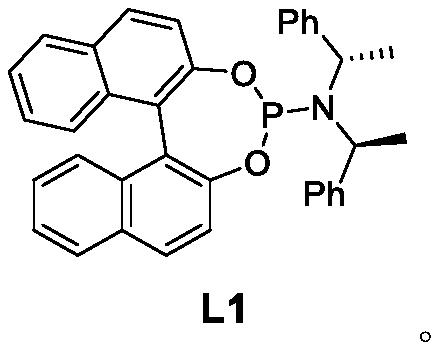
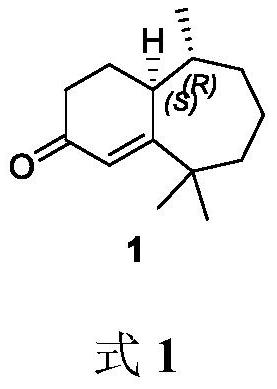
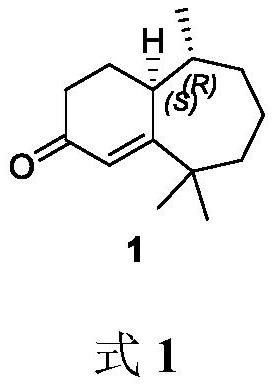
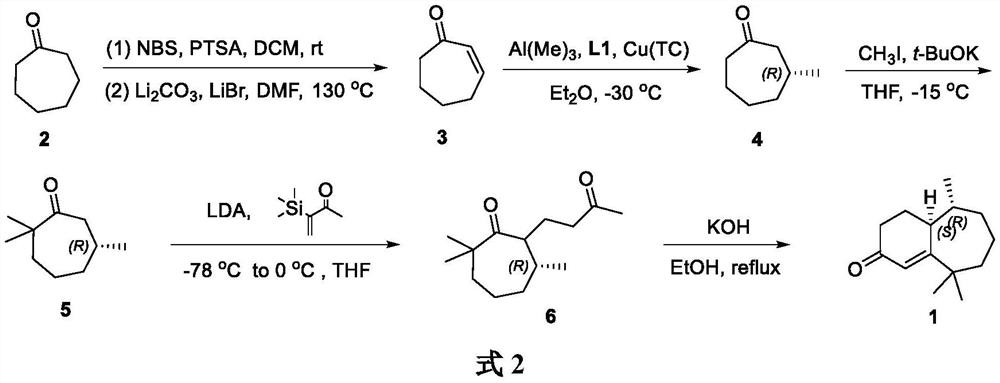
Method for synthesizing flea beetle aggregation pheromone
ActiveCN113773180AOrganic compound preparationOrganic chemistry methodsCycloheptanoneLithium bromide
The invention belongs to the technical field of green pesticides, and discloses a novel method for synthesizing flea beetle aggregation pheromone. The method comprises the following steps of: with cycloheptanone (2) taken as an initial raw material, carrying out bromination reaction on the cycloheptanone and NBS (N-bromosuccinimide), carrying out elimination reaction under the alkaline condition of lithium bromide and lithium carbonate to obtain cycloheptenone 3, carrying out asymmetric Michael addition reaction under the catalysis of copper to prepare chiral methyl cycloheptanone 4; and making the chiral methyl cycloheptanone 4 react with methyl iodide to obtain trimethyl cycloheptanone 5. carrying out aldol condensation reaction on the trimethyl cycloheptanone 5 and trimethylsilyl ketene under the catalysis of LDA to prepare diketone 6; and finally carrying out Robinson cyclization reaction to obtain flea beetle aggregation pheromone 1. The asymmetric Michael addition reaction is utilized for the first time to construct the chiral methyl of the flea beetle aggregation pheromone, and the method has the advantages of being simple in synthetic route, easy to operate and the like.
Owner:CHINA AGRI UNIV
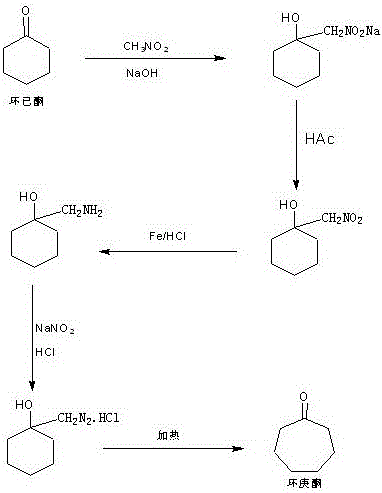
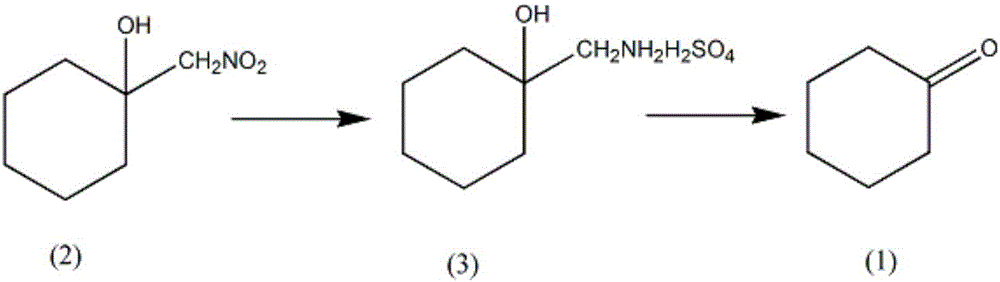
Synthesis method of intermediate cycloheptanone of guanethidine sulfate drug
InactiveCN106431856AReduce intermediate linksLow reaction temperatureOrganic compound preparationCarbonyl compound preparationSynthesis methodsWater vapor
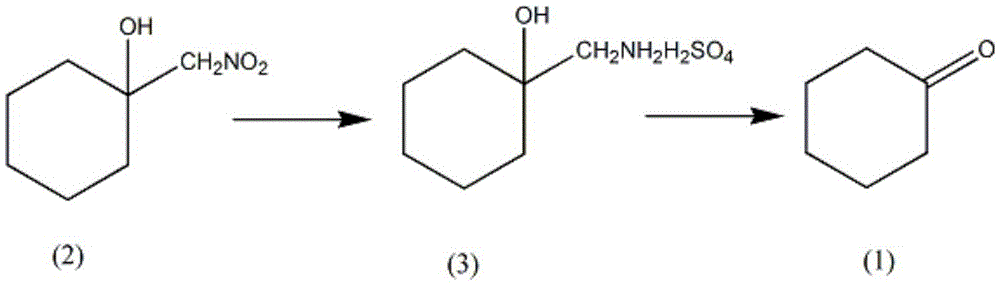
A synthesis method of intermediate cycloheptanone of a guanethidine sulfate drug comprises the steps as follows: (i) 500-600 ml of water, 1.5 mol of metal powder and a phosphoric acid solution with the mass percentage being 40% -50% are added to a reaction vessel equipped with a stirrer, a thermometer and a dropping funnel, the temperature of the solution is controlled to be 60-70 DEG C, 1.2 mol of nitromethylcyclohexanol is added dropwise, the temperature of the solution is maintained to be 40-50 DEG C after addition, 30 mL of a sulfuric acid solution with the mass percentage being 40%-45% is added at an interval of 30 min and is added 3 times altogether, the pH of the solution is maintained to be 3-4 for 1 h, the filtrate is an aminomethyl cyclohexanol sulfate solution, and the filter cake is washed with a solvent and used for the next batch; (ii) pH of the solution is adjusted with sulfuric acid to be 2-3, the solution temperature is reduced to 2-5 DEG C, 1.5-2 mol of a sodium sulfite solution is added slowly and dissolved in 200 ml of water, the temperature is increased to 30-35 DEG C, distillation is performed through steam until no oily substances are distilled off, an oil layer is separated, a water layer is extracted with a solvent for 10-15 times, the oil layer and the solvent layer are combined, a solvent is distilled off under atmospheric pressure, distilling under reduced pressure is performed, a fraction at 65-70 DEG C is collected, and cycloheptanone is obtained.
Owner:厦门莱恩斯特信息科技有限公司
Method of preparing alpha,alpha'-di(substituted benzylidene)cyclone ultraviolet radiation absorbent
InactiveCN100497293COrganic compound preparationOther chemical processesAcetic acidUltraviolet radiation
The invention relates to a method for preparing a class of ultraviolet absorber of alpha, alpha'-di(substituted benzilidene)ring ketone, in which various alpha, alpha'-di(substituted benzilidene)ring ketone compounds, alpha, alpha'-di(substituted benzilidene)cyclohexanones compounds and alpha, alpha'-di(substituted benzilidene)cycloheptanone compounds are generated through taking mixed solution of concentrated sulfuric acid with acetic as reaction solution and reacting under room temperature condition. The ultraviolet absorber of alpha, alpha'-di(substituted benzilidene)ring ketone compounds has stable chemical properties, can intensely absorb ultraviolet, in which its absorption range is 230-400n, and it can be used as wide spectrum ultraviolet absorbent.
Owner:ZHEJIANG UNIV
A kind of method for synthesizing flea beetle aggregation pheromone
ActiveCN113773180BOrganic compound preparationOrganic chemistry methodsCycloheptanoneLithium bromide
Owner:CHINA AGRI UNIV
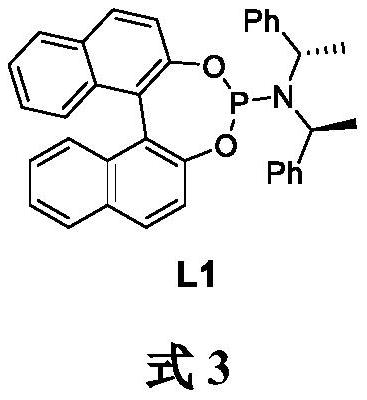
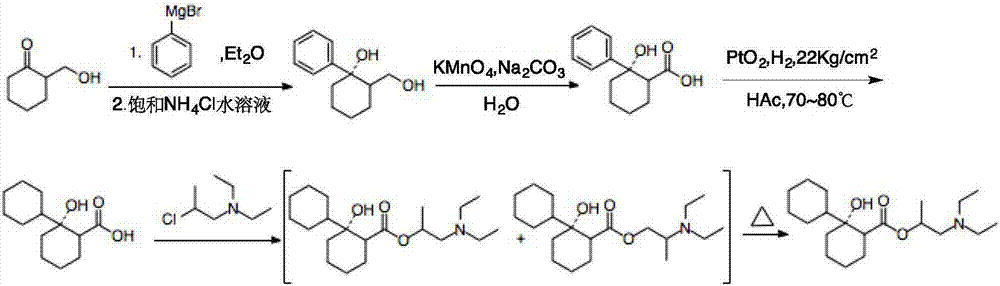
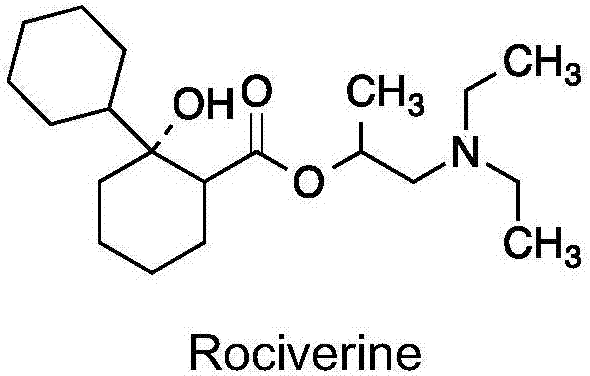
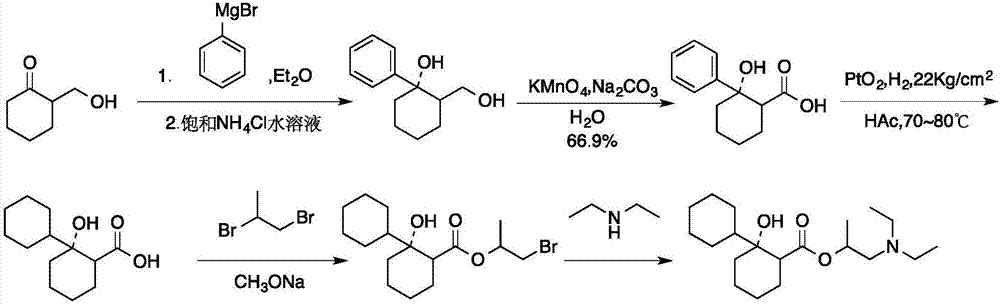
Preparation method of 2-diethylamino-1-methylethyl-7-cyclohexyl-7-oxoheptanoate
ActiveCN107286031AEasy to handleOrganic compound preparationCarboxylic preparation by oxidationSynthesis methodsCycloheptanone
The invention provides a compound 2-diethylamino-1-methylethyl-7-cyclohexyl-7-oxo-heptanoate (1) and a synthesis method thereof. The compound 1 adopts a structural formula as shown in the specification. The invention further provides the application of the compound 1 as an impurity reference substance for monitoring synthesis of an active pharmaceutical ingredient of rociverine. By taking cycloheptanone as a starting material, the compound 1 is synthesized through a four-step reaction; the obtained product is simple in posttreatment of each step, the product is convenient and easy to obtain, the compound 1 is suitable for being synthesized on a large scale, and a relevant substance or the impurity reference substance are provided for quality research on the synthesis of the active pharmaceutical ingredient of the rociverine.
Owner:SHANGHAI INST OF PHARMA IND +1
Dibenzoxepanone compounds and preparation method and use thereof
ActiveCN108485987BPotent anti-Vibrio parahaemolyticus activityAntibacterial agentsOrganic active ingredientsBiotechnologyCycloheptanone
Owner:YANGZHOU UNIV
Starch / C4~C8 ring graft copolymer and prep. and use thereof
InactiveCN1194023CWill not cause secondary pollutionImprove hydrophobicityPolymer sciencePtru catalyst
Owner:SICHUAN UNIV
Synthetic method of guanethidine subplate drug intermediate cycloheptanone
InactiveCN105461524AReduce intermediate linksLow reaction temperatureOrganic compound preparationAmino compound preparationWater vaporSulfite salt
The invention provides a synthetic method of guanethidine subplate drug intermediate cycloheptanone. The synthetic method comprises the following steps: (1) adding 500 to 600ml of water, 1.5mol of metal powder, a phosphoric acid solution with the mass fraction of 40 to 50 percent into a reaction vessel provided with a stirrer, a thermometer and a dropping funnel, wherein the solution temperature is controlled to be 60 to 70 DEG C, dropwise adding 1.2mol of nitromethylcycloheanol, after adding, maintaining the solution temperature at 40 to 50 DEG C, adding 30mL of sulfuric acid solution with the mass fraction of 40 to 45 percent for 3 times every 30min, and maintaining the solution pH at 3 to 4 for 1h, wherein filter liquor is aminomethlcycloheanol sulfate liquor, and a filter liquor cake is used for the next batch after being washed by using a solvent; (2) regulating the solution pH to 2 to 3 by using sulfuric acid, reducing the solution temperature to 2 to 5 DEG C, slowly adding and dissolving 1.5 to 2mol of sodium sulfite in 200ml of water, then heating up to 30 to 35 DEG C, distilling by using steam till a non-oily matter is distilled off, separating out an oil layer, extracting a water layer for 10 to 15 times by a solvent, combining the oil layer and a solvent layer, distilling off the solvent at normal pressure, then performing reduced pressure distillation, and collecting 65 to 70 DEG C cut fraction to obtain the cycloheptanone.
Owner:CHENGDU ZHONGHENG HUATIE TECH CO LTD
Compound for the treatment of human liver cancer and method for synthesizing it
The compound “2-((4-nitrophenyl)amino)-7,8,9,10-tetrahydro cyclohepta[4,5]thieno[2,3-d][1,3,4]thiadiazolo[3,2-a]pyrimidin-11(6H)-one” and method of synthesizing it, wherein the compound is effective to inhibit the growth and proliferation of human liver cancer cells HepG2. The compound has a higher efficiency to inhibit the growth and proliferation of these cells as it has an inhibitory concentration value (IC50) of 0.7 μg, compared to reference medication Doxorubicin that has an (IC50) value of 1.2 μg. It further surpasses that reference medication at all tested concentrations. The method includes the steps of: preparing a first compound of cycloheptanone, ethylcyanoacetate, sulfur, ethanol and diethylamine; preparing a second compound by heating of the first compound with excess of hydrazine hydrate in absolute ethanol as solvent; preparing a third compound by heating the second compound with carbon disulphide in dry pyridine; and preparing the compound of the invention by reacting the third compound with 4-nitrophenylisothiocyanate in dry N,N-methylformamide as solvent.
Owner:ALGHAMDI ZAINAB SAEED
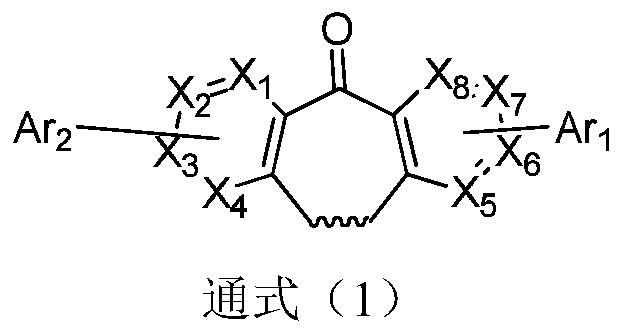
Method for preparing 8-chloro-10, 11-dihydro-4-aza-5H-dibenzo[a, d]-5-cycloheptanone
InactiveCN1640869AAvoid generatingGood effectOrganic chemistryChemical reactionSedating Antihistamines
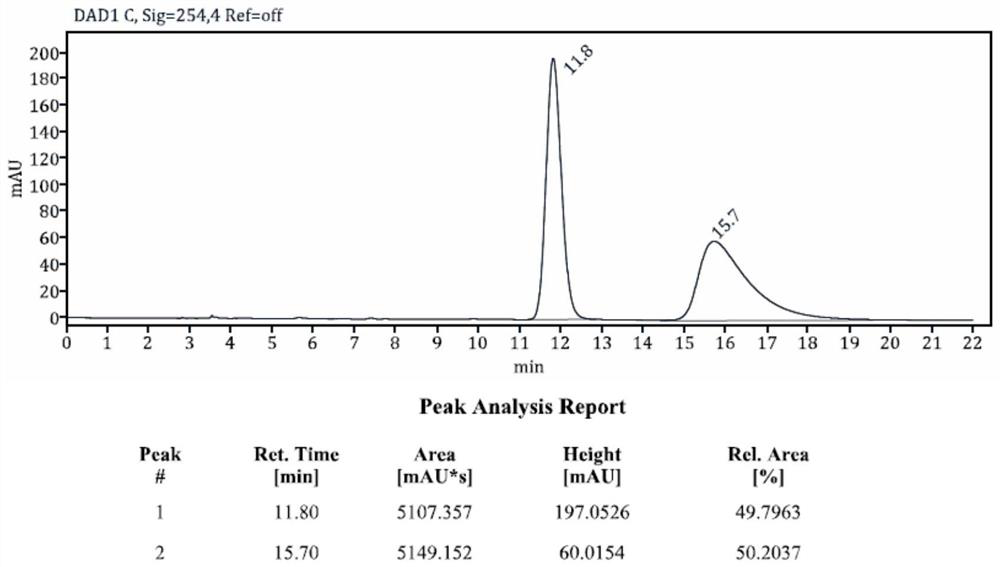
The present invention relates to the new preparation process of 8-chloro-10, 11-dihydro-4-aza-5H-diphenyl[a, d]-5-cycloheptanone as the important intermediate of 2G non-calmative antihistamine loratadine. The preparation process includes successively the chemical reaction of the compound in expression (2) and phosphorus-containing reagent in solvent, the direct catalytic Friedel-Crafts reaction, and final hydrolysis to obtain target product in the same reactor. The synthesis process of the present invention has less reaction steps and simple operation, high product purity, and is suitable for industrial production.
Owner:上海康鸣高科技有限公司 +1
Camphor-based ketoxime fluorescent probe for detecting hypochlorous acid as well as preparation method and application of camphor-based ketoxime fluorescent probe
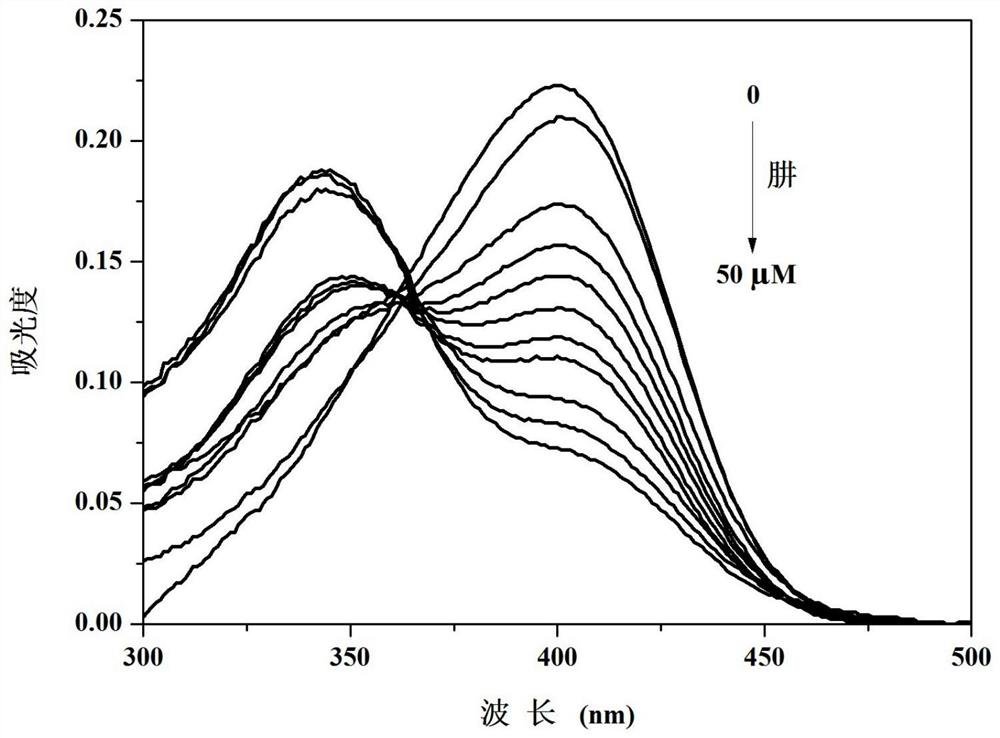
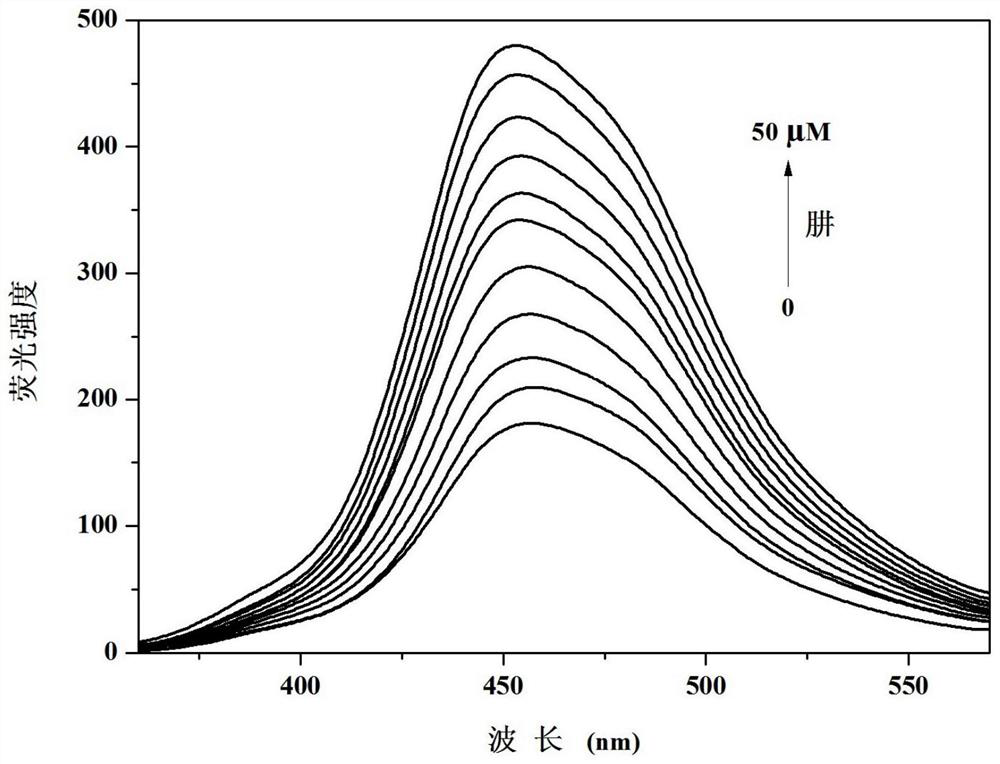
PendingCN114478308ADetection of hypochlorous acid contentGood choiceOximes preparationFluorescence/phosphorescenceFluoProbesHydroxylamine
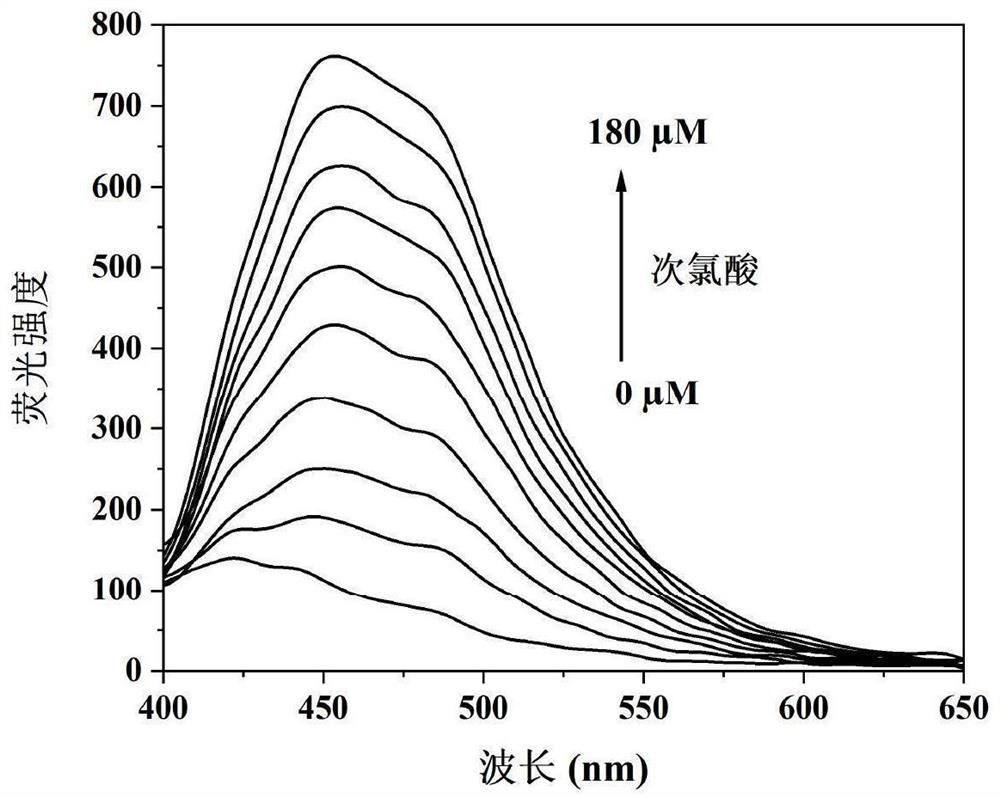
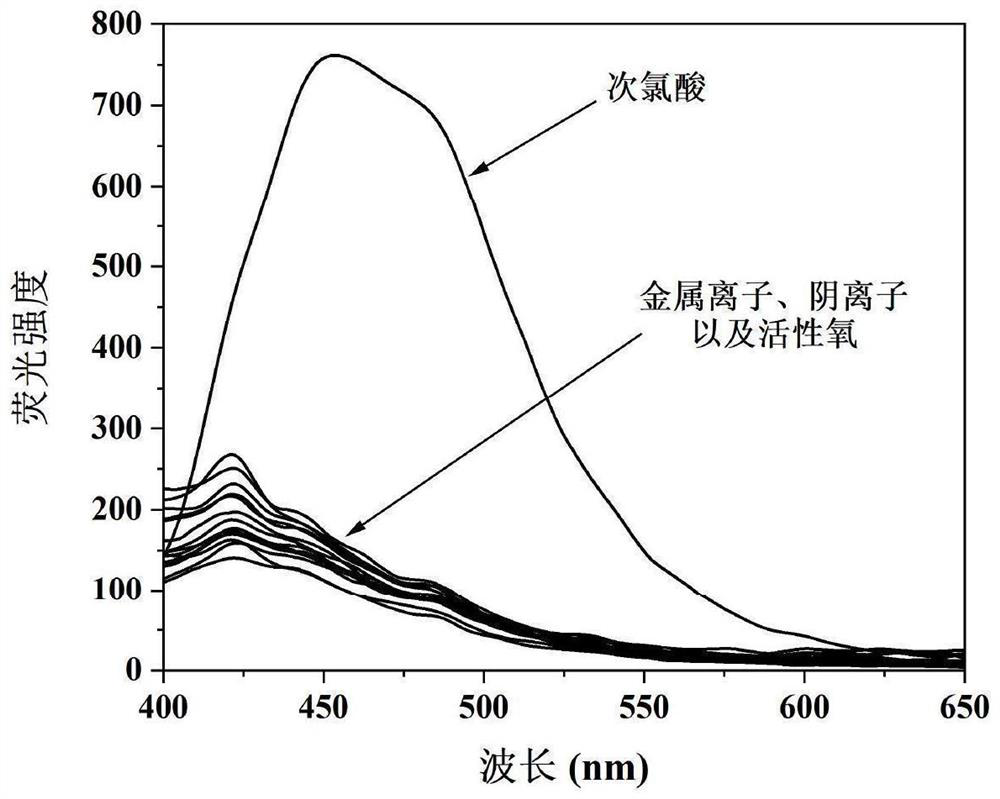
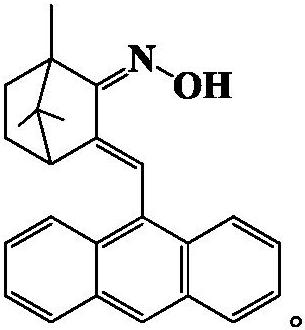
The invention discloses a camphor-based ketoxime fluorescent probe for detecting hypochlorous acid as well as a preparation method and application of the camphor-based ketoxime fluorescent probe. The fluorescent probe is 3-(9-anthracene methylene)-1, 7, 7-trimethyl bicyclo [2.2. 1] heptyl-2-ketoxime, and the structural formula of the fluorescent probe is shown in the description. According to the invention, 3-(9-anthracene methylene)-1, 7, 7-trimethyl bicyclo [2.2. 1] heptyl-2-ketone is used as a raw material, and the 3-(9-anthracene methylene)-1, 7, 7-trimethyl bicyclo [2.2. 1] heptyl-2-ketoxime is obtained by carrying out condensation reaction on the 3-(9-anthracene methylene)-1, 7, 7-trimethyl bicyclo [2.2. 1] heptyl-2-ketone and hydroxylamine hydrochloride. The compound can selectively react with hypochlorous acid, and the fluorescence color of a probe solution is changed from colorless to bright blue under the irradiation of 365nm ultraviolet light, so that the compound can be used as a fluorescent probe for selectively detecting hypochlorous acid, the probe can quickly and sensitively detect the content of hypochlorous acid in the solution, the detection range is 0-180 mu M, and the application prospect is broad. The detection limit is as low as 1.18 * 10 <-7 > M, the detection time is less than 5 s, and the method has a good application prospect.
Owner:NANJING FORESTRY UNIV
Compound for inhibiting the growth and proliferation of human liver cancer cells and method for synthesizing it
ActiveUS20160280717A1Promote growthReducing propagationOrganic chemistryHydrazine compoundCycloheptanone
The compound “2-((4-nitrophenyl)amino)-6,7,8,9-tetrahydro-3H-cyclohepta[4,5]thieno-[2,3-d]pyrimidin-4(5H)-one” and method of synthesizing it, wherein the compound is effective to inhibit the growth and proliferation of human liver cancer cells HepG2. The compound has an inhibitory concentration value (IC50) of 0.7 μg, compared to reference medication Doxorubicin that has an (IC50) value of 1.2 μg. It further surpasses that reference medication Doxorubicin at all tested concentraions. The method includes the steps of: preparing a first compound of cycloheptanone, ethylcyanoacetate, sulfur, ethanol and diethyl amine; preparing a second compound by heating of the first compound with excess of hydrazine hydrate in absolute ethanol as solvent; and preparing the effective compound of the invention by reaction of the second chemical compound with 4-nitrophenylisothiocyanate in dry dimethylformamide as solvent.
Owner:ALGHAMDI ZAINAB SAEED
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![6,7-dihydro-5H-diphenyl[a,c]cyclohepta-5-ketone compound and preparation method thereof 6,7-dihydro-5H-diphenyl[a,c]cyclohepta-5-ketone compound and preparation method thereof](https://images-eureka.patsnap.com/patent_img/bca9c472-92cf-44f9-a0e0-1cc31f468d9d/1.PNG)
![6,7-dihydro-5H-diphenyl[a,c]cyclohepta-5-ketone compound and preparation method thereof 6,7-dihydro-5H-diphenyl[a,c]cyclohepta-5-ketone compound and preparation method thereof](https://images-eureka.patsnap.com/patent_img/bca9c472-92cf-44f9-a0e0-1cc31f468d9d/64266DEST_PATH_77703DEST_PATH_IMAGE006.PNG)
![6,7-dihydro-5H-diphenyl[a,c]cyclohepta-5-ketone compound and preparation method thereof 6,7-dihydro-5H-diphenyl[a,c]cyclohepta-5-ketone compound and preparation method thereof](https://images-eureka.patsnap.com/patent_img/bca9c472-92cf-44f9-a0e0-1cc31f468d9d/81441DEST_PATH_RE-DEST_PATH_IMAGE014.PNG)
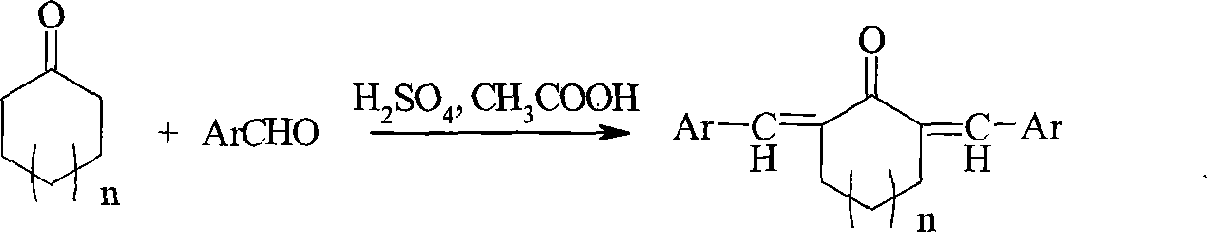
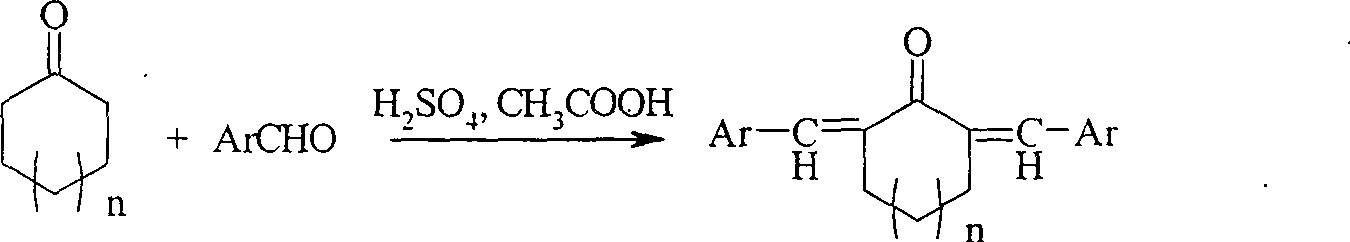

![Method for preparing 8-chloro-10, 11-dihydro-4-aza-5H-dibenzo[a, d]-5-cycloheptanone Method for preparing 8-chloro-10, 11-dihydro-4-aza-5H-dibenzo[a, d]-5-cycloheptanone](https://images-eureka.patsnap.com/patent_img/d9996c06-ade4-4ec7-b05f-a10cbf9230a4/A2004100158830002C1.PNG)
![Method for preparing 8-chloro-10, 11-dihydro-4-aza-5H-dibenzo[a, d]-5-cycloheptanone Method for preparing 8-chloro-10, 11-dihydro-4-aza-5H-dibenzo[a, d]-5-cycloheptanone](https://images-eureka.patsnap.com/patent_img/d9996c06-ade4-4ec7-b05f-a10cbf9230a4/A20041001588300031.PNG)
![Method for preparing 8-chloro-10, 11-dihydro-4-aza-5H-dibenzo[a, d]-5-cycloheptanone Method for preparing 8-chloro-10, 11-dihydro-4-aza-5H-dibenzo[a, d]-5-cycloheptanone](https://images-eureka.patsnap.com/patent_img/d9996c06-ade4-4ec7-b05f-a10cbf9230a4/A20041001588300032.PNG)