A camphor-based fluorescent probe for detecting hydrazine and its preparation method
A fluorescent probe and camphor-based technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of long response time, poor water solubility, low sensitivity, etc., and achieve good practical value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation of camphor-based fluorescent probe, reaction formula is as follows:
[0033]
[0034] Specific steps are as follows:
[0035] 1) Preparation of 3-(4'-dimethylaminobenzylidene)-1,7,7-trimethylbicyclo[2.1.1]heptan-2-one:
[0036] Put 6mmol camphor, 6.5mmol p-dimethylaminobenzaldehyde, 12mmol sodium ethoxide and 30mL ethanol in a three-necked flask equipped with a stirrer, a thermometer and a reflux condenser in sequence, and heat it to reflux at 80-90°C to react for 5 hours to The conversion rate of camphor is more than 95% (GC tracking detection). The reaction solution was distilled to recover ethanol, then 25 mL of ethyl acetate was added, and washed with saturated brine until neutral. The organic phase was dried over anhydrous sodium sulfate, filtered, and concentrated to recover the solvent to obtain 3-(4'-dimethylaminobenzylidene)-1,7,7-trimethylbicyclo[2.1.1]hept-2 - crude ketones. The crude 3-(4′-dimethylaminobenzylidene)-1,7,7-trimethylbicyc...
Embodiment 2
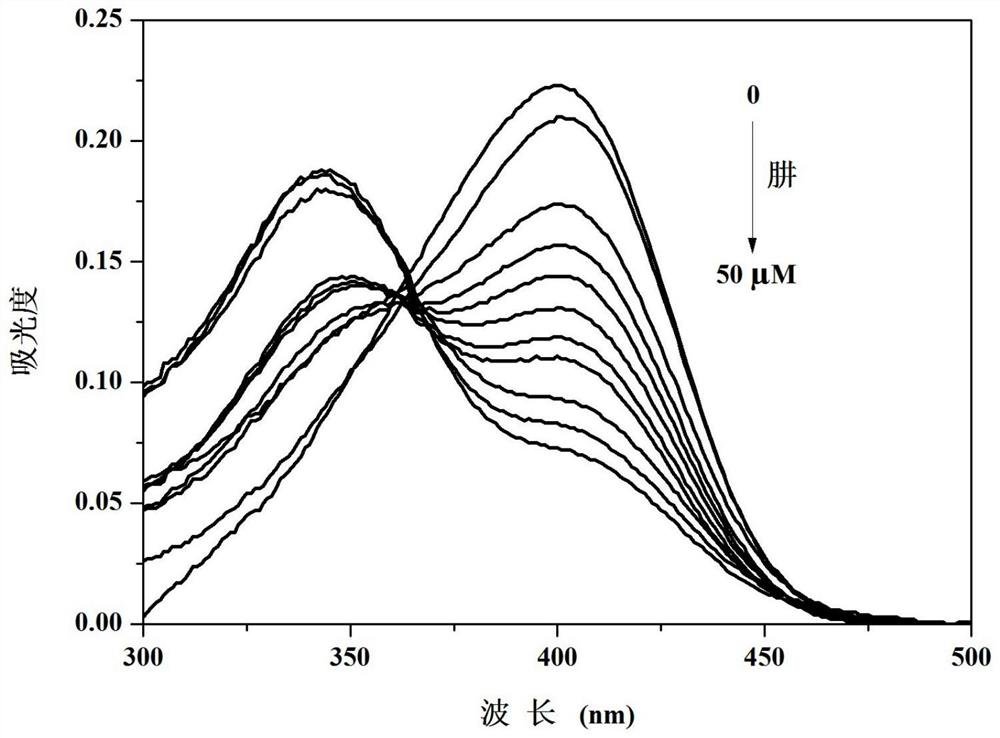
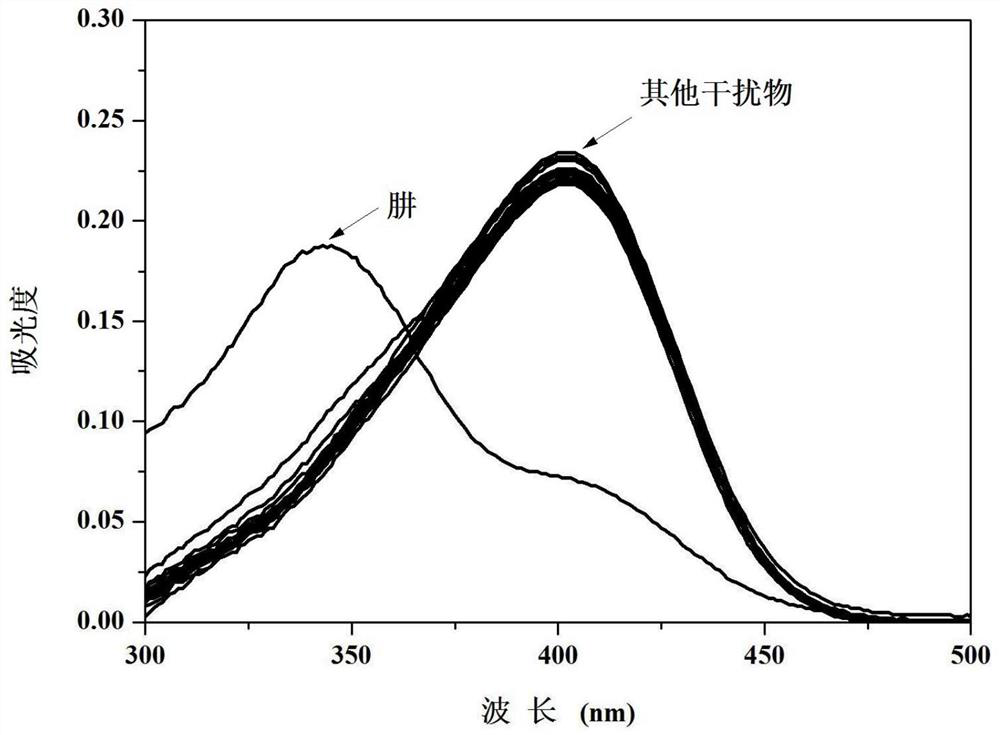
[0040] Compound 4-(4′-dimethylaminophenyl)-8,9,9-trimethyl-5,6,7,8-tetrahydro-5,8-endomethylene in different concentrations of hydrazine solutions The Ultraviolet Spectrum Properties of Quinazolin-2-amine
[0041] 4-(4'-dimethylaminophenyl)-8,9,9-trimethyl-5,6,7,8-tetrahydro-5,8-methanoquinazole obtained in Example 1 Phenyl-2-amine was formulated as 1 x 10 -5 M of PBS buffer solution (10mM, pH=7.4, 10% (v / v) ethanol), hydrazine is dissolved in the PBS buffer solution and is made into concentration and is 0,5,10,15,20,25,30,35, 40, 45, 50 μM solutions. Measured different concentrations of hydrazine to 4-(4'-dimethylaminophenyl)-8,9,9-trimethyl-5,6,7,8-tetrahydro-5,8-methanoquinone The ultraviolet absorption spectrum of oxazolin-2-amine, such as figure 1 shown. The results showed that the ultraviolet absorption of the compound at 400nm decreased obviously, while the absorption around 343nm increased obviously, which indicated that the compound could react with hydrazine.
Embodiment 3
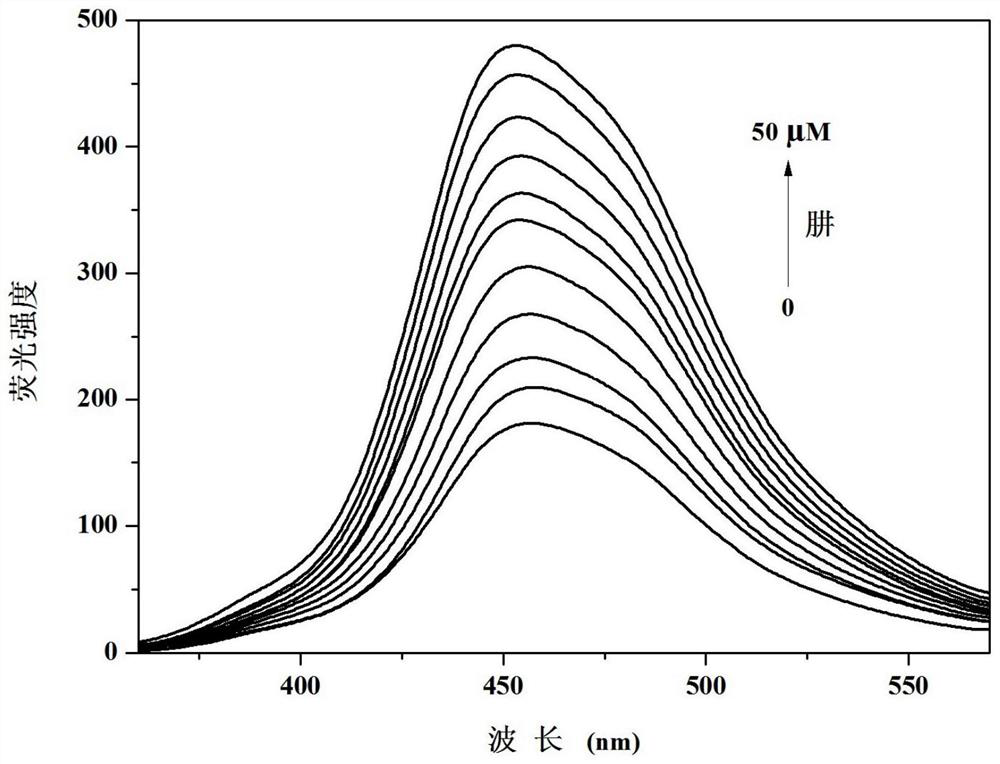
[0043] Compound 4-(4′-dimethylaminophenyl)-8,9,9-trimethyl-5,6,7,8-tetrahydro-5,8-endomethylene in different concentrations of hydrazine solutions Fluorescence Spectrum Properties of Quinazolin-2-amine
[0044] 4-(4'-dimethylaminophenyl)-8,9,9-trimethyl-5,6,7,8-tetrahydro-5,8-methanoquinazole obtained in Example 1 Phenyl-2-amine was formulated as 1 x 10 -5 M of PBS buffer solution (10mM, pH=7.4, 10% (v / v) ethanol), hydrazine is dissolved in the PBS buffer solution and is made into concentration and is 0,5,10,15,20,25,30,35, 40, 45, 50 μM solutions. Measured different concentrations of hydrazine to 4-(4'-dimethylaminophenyl)-8,9,9-trimethyl-5,6,7,8-tetrahydro-5,8-methanoquinone The fluorescence emission spectrum of oxazolin-2-amine, as figure 2 shown. The results showed that the fluorescence intensity of the compound at 451nm was significantly enhanced, indicating that the compound could react with hydrazine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com