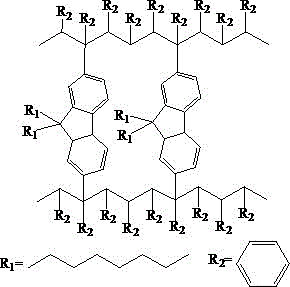
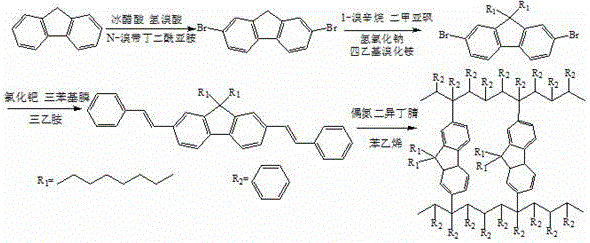
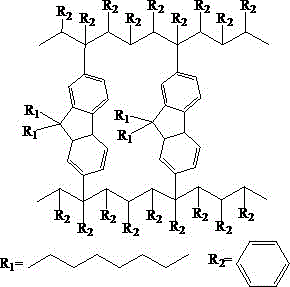
Non-conjugated polymer blue light material containing fluorene main chain and preparation method thereof
A technology of non-conjugated polymer and blue light material is applied in the field of non-conjugated polymer blue light material containing fluorene main chain and its preparation, which can solve the problems such as price and environmental pollution restricting development potential, reducing device efficiency, and complex process. Achieve the effects of excellent solubility, convenient film formation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 1) Synthesis of 2,7-dibromofluorene
[0021] Add 1.5 g fluorene and 3 g N-bromosuccinimide to a 100 mL three-neck flask, 20 mL solvent glacial acetic acid, nitrogen protection, stir at 25 °C for 40 min, and add 0.5 mL 48% hydrogen after the solid is completely dissolved Bromic acid, again filled with nitrogen protection. Stir for 12 h at room temperature in the dark, and stop the reaction when the color of the solution changes from orange to yellow. Add a large amount of water for suction filtration and washing, and recrystallize the solid with a mixture of ethanol and chloroform (V ethanol: V chloroform = 2:1) to obtain a white needle-like product.
[0022] 2) Synthesis of 9,9-dioctyl-2,7-dibromofluorene
[0023] In a 100 mL three-necked flask, 2 g of 2,7-dibromofluorene, 2.66 g of bromooctane, 0.0704 g of tetraethylammonium bromide, 2.4 g of sodium hydroxide, and 20 mL of solvent dimethyl sulfoxide were sequentially added. Filled with nitrogen protection. Stir for...
Embodiment 2
[0029] 1) Synthesis of 2,7-dibromofluorene
[0030] Add 1.5 g fluorene and 4.5 g N-bromosuccinimide to a 100 mL three-neck flask, 20 mL solvent glacial acetic acid, nitrogen protection, stir at 40°C for 30 min, and add 0.5 mL 48% hydrogen bromide after the solid is completely dissolved Acid, nitrogen protection again. Stir at 40°C for 7 h in the dark, and stop the reaction when the color of the solution changes from orange-red to yellow. Add a large amount of water for suction filtration and washing, and recrystallize the solid with a mixture of ethanol and chloroform (V ethanol: V chloroform = 2:1) to obtain a white needle-like product.
[0031] 2) Synthesis of 9,9-dioctyl-2,7-dibromofluorene
[0032] Into a 100 mL three-necked flask, 2 g of 2,7-dibromofluorene, 3 g of bromooctane, 0.1 g of tetraethylammonium bromide, 3 g of sodium hydroxide, and 20 mL of solvent dimethyl sulfoxide were sequentially added. Filled with nitrogen protection. Stir for 16 h at 40 °C in the dar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com