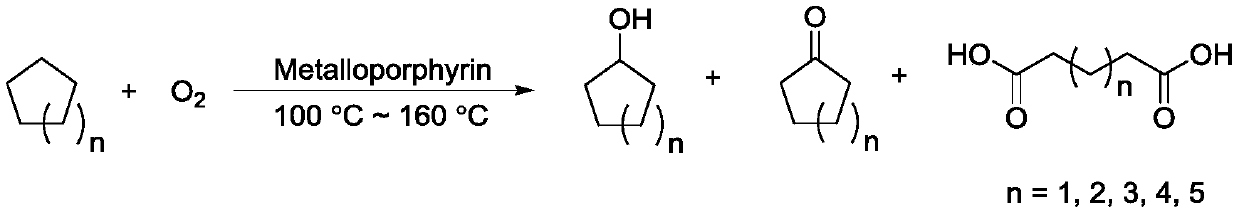
Cycloalkane catalytic oxidation method promoted by iron porphyrin
A technology for catalytic oxidation and naphthenic hydrocarbons, applied in chemical instruments and methods, hydrocarbon oxidation to prepare oxygenated compounds, catalytic reactions, etc., can solve the problems of low product selectivity, poor environmental protection, complicated operation, etc. Low dosage and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] In a 100mL stainless steel autoclave with a tetrafluoroethylene liner, dissolve 1.5mg of 5,10,15,20-tetrakis(p-chlorophenyl)porphyrin cobalt(II) in 50.0g of cyclohexane, and close the autoclave kettle. Stir to raise the temperature to 120°C, pass O 2 to 1.4MPa, at 120°C, 1.4MPa O 2 The reaction was stirred under pressure for 8.0 h, stirred and cooled to room temperature in an ice-water bath. Open the autoclave, filter the obtained reaction mixture, wash the obtained solid with 3 × 5mL cyclohexane, and dry it under vacuum at 60°C, which is adipic acid. The yield of HPLC analysis is 0.32%, and the yield of cyclohexanol is 1.34% by GC analysis of the filtrate. %, cyclohexanone yield 1.43%.
Embodiment 2
[0023] In a 100mL stainless steel autoclave with a tetrafluoroethylene liner, dissolve 1.5mg of 5,10,15,20-tetrakis(p-chlorophenyl)porphyrin iron (II) in 50.0g of cyclohexane, and close the high pressure kettle. Stir to raise the temperature to 120°C, pass O 2 to 1.4MPa, at 120°C, 1.4MPa O 2 The reaction was stirred under pressure for 8.0 h, stirred and cooled to room temperature in an ice-water bath. Open the autoclave, filter the obtained reaction mixture, wash the obtained solid with 3×5mL cyclohexane, and dry it under vacuum at 60°C, which is adipic acid. The yield of HPLC analysis is 0.01%, and the yield of cyclohexanol is 0.22% by GC analysis of the filtrate. %, cyclohexanone yield 0.35%.
Embodiment 3
[0025] In a 100 mL stainless steel autoclave with a tetrafluoroethylene liner, 1.5 mg of 5,10,15,20-tetrakis(p-chlorophenyl)porphyrin cobalt(II) and 1.5 mg of 5,10,15,20- Tetrakis(p-chlorophenyl)porphyrin iron(II) was dissolved in 50.0 g of cyclohexane, and the autoclave was closed. Stir to raise the temperature to 120°C, pass O 2 to 1.4MPa, at 120°C, 1.4MPa O 2 The reaction was stirred under pressure for 8.0 h, stirred and cooled to room temperature in an ice-water bath. Open the autoclave, filter the obtained reaction mixture, wash the obtained solid with 3 × 5mL cyclohexane, and dry it under vacuum at 60°C, which is adipic acid. The yield of HPLC analysis is 0.15%, and the yield of cyclohexanol is 1.41% by GC analysis of the filtrate. %, cyclohexanone yield 1.38%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com