Patents
Literature
41 results about "Cyclononane" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cyclononane is an alicyclic hydrocarbon consisting of a ring of nine carbon atoms. Its molecular formula is C₉H₁₈.
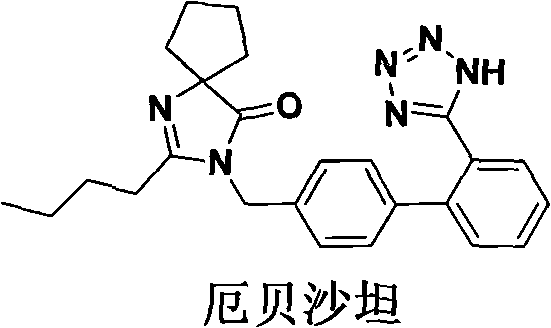
Synthetic route and preparation method of irbesartan
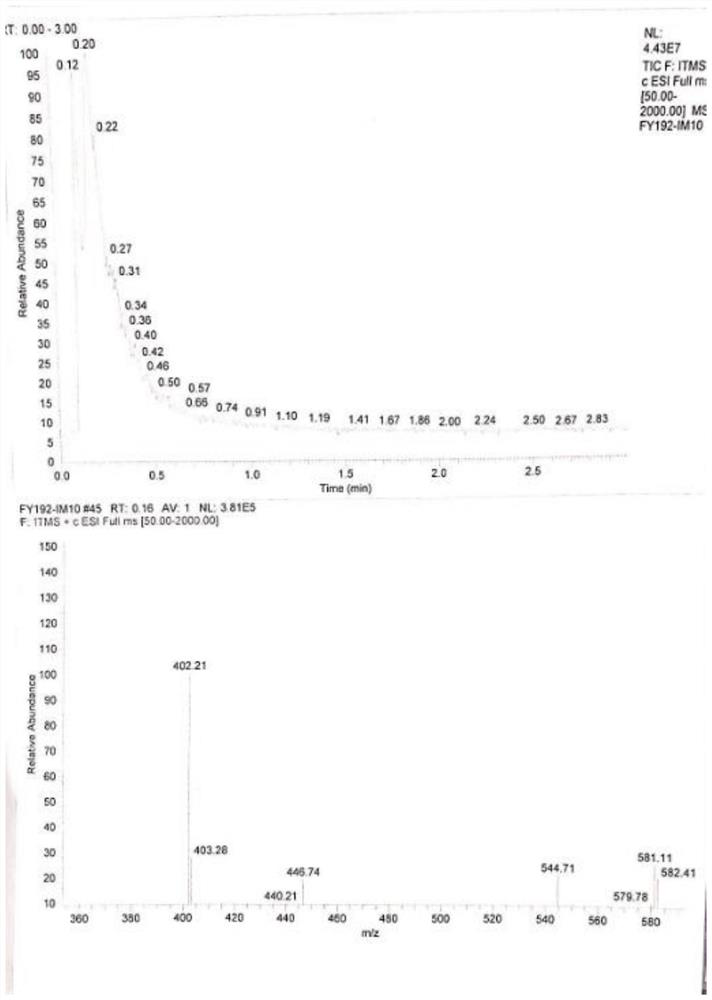
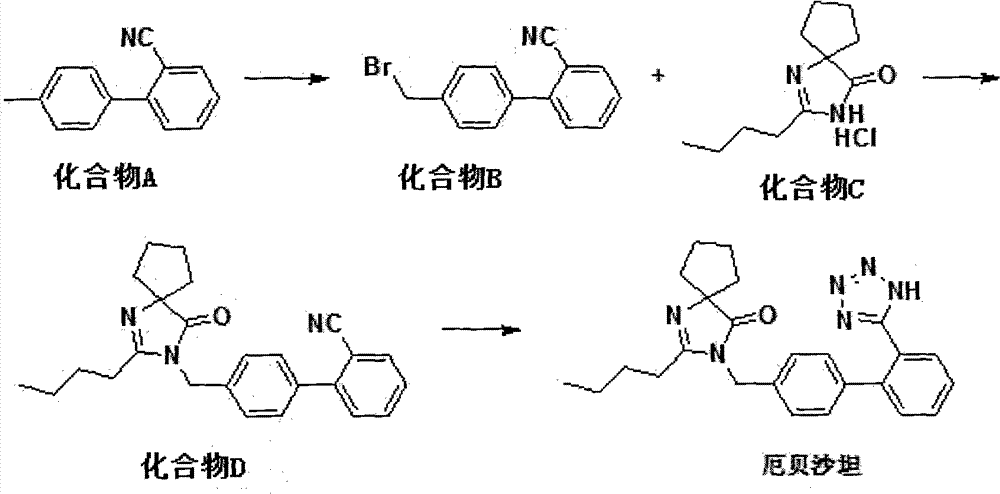
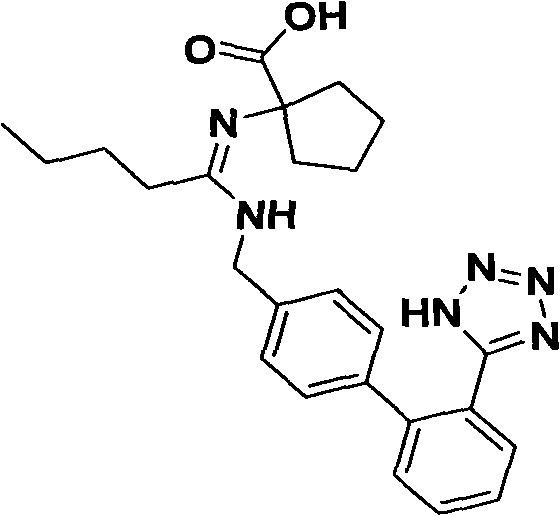
The invention relates to a synthetic route and a preparation method of irbesartan. The method comprises three steps: (1) reacting a compound I (2-cyano-4'-methyl diphenyl), an inorganic salt oxidant, and an inorganic salt reductant in dichloromethane and water to form a compound IRB-02 (2-cyano-4'-bromomethylbiphenyl); (2) reacting a compound IRB-02, a compound IRB-01 (2-butyl-1, 3-diaza spiro [4.4] nonane-1-vinyl-4-ketone hydrochloride), tetrabutylammonium bromide and inorganic alkali in dichloromethane and water to obtain a compound IRB-03 (2-butyl-3-[(2-cyano biphenyl-4-base)methyl]-1,3-diaza spiro [4.4] nonane-1-vinyl-4-ketone); and (3) reacting a compound IRB-03, tetrabutylammonium bromide, zinc chloride and sodium azide in toluene to obtain the irbesartan.
Owner:珠海保税区丽珠合成制药有限公司
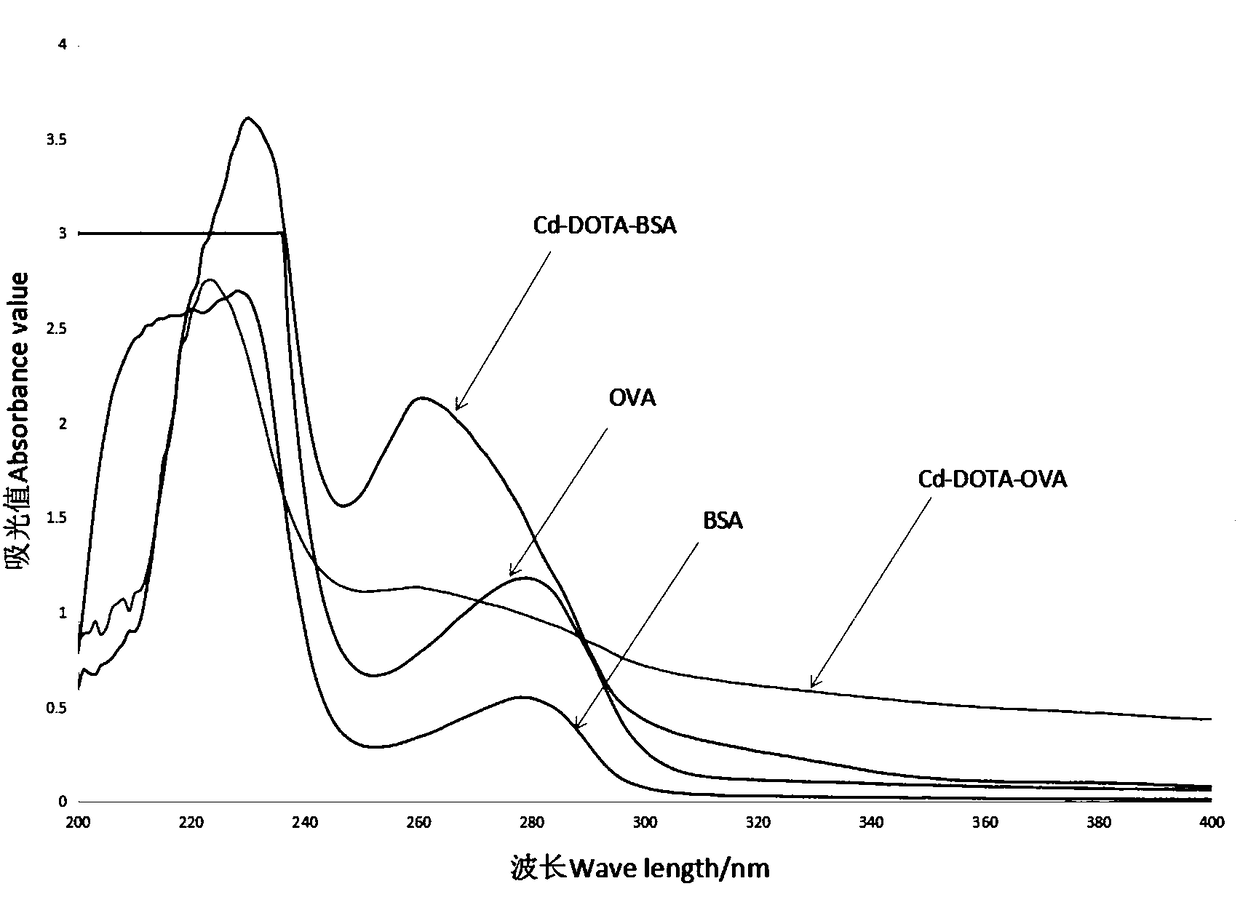
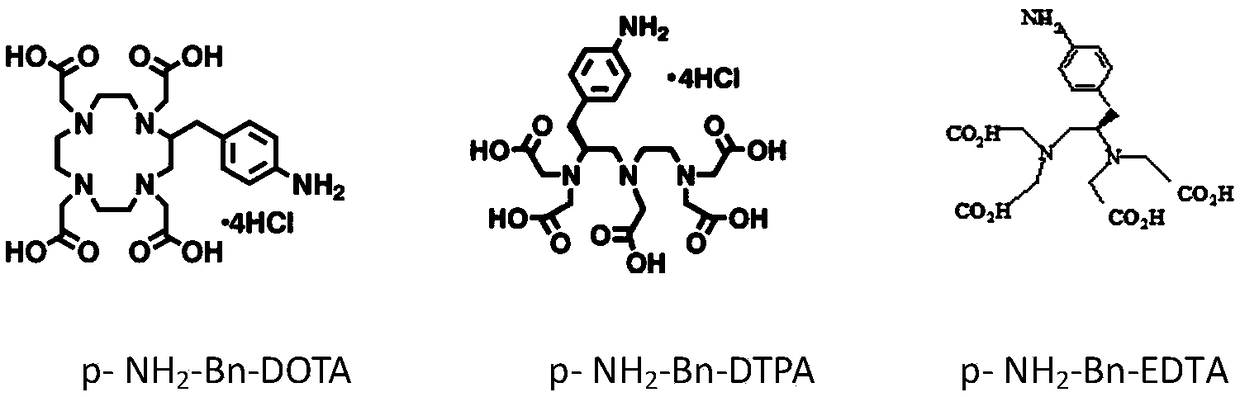
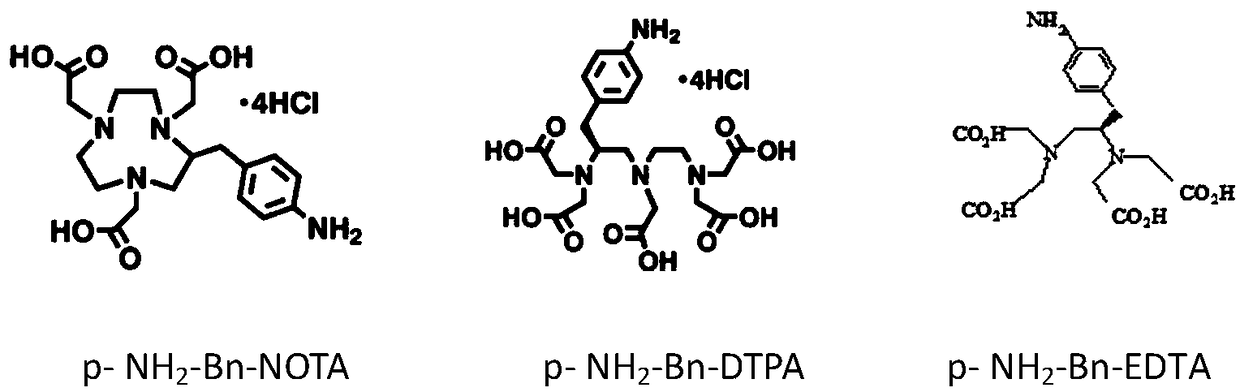
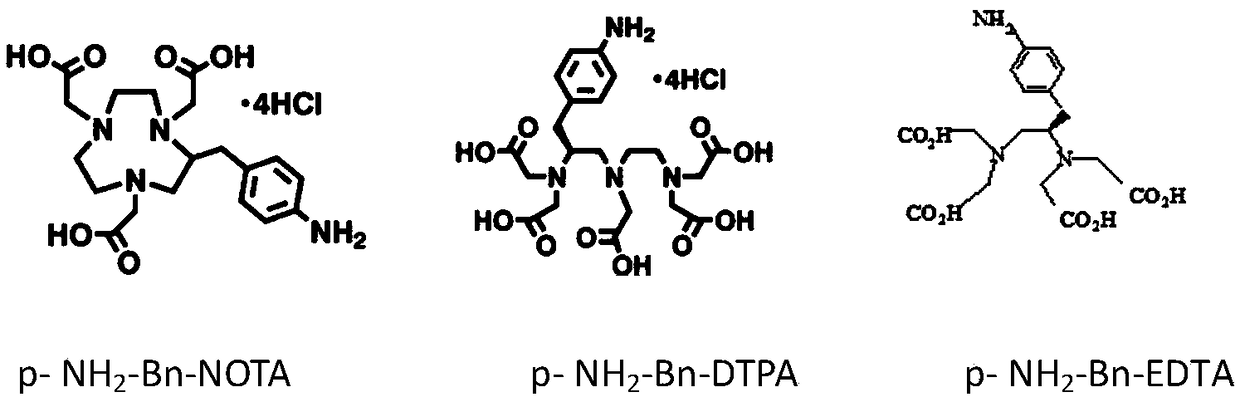
Preparation method of heavy metal cadmium artificial antigen and application of DOTA in preparation of heavy metal cadmium artificial antigen reagent
The invention discloses a preparation method of heavy metal cadmium artificial antigen, which applies 2-S-(4- aminobenzene)-1, 4, 7, 10 tetraazacyclo cyclononane-1, 4, 7, 10-acetate tetrahydrate(p-NH2-Bn-DOTA, DOTA for short) as cheating agent, and couples cadmium ion chelating agent composite with carrier protein bovine serum albumin BSA or egg albumin OVA; thus the artificial antigen is prepared. The method adds the sodium borohydride processing step on the basis of traditional method, and improves the coupling efficiency and antiserum titre. The invention further discloses an application ofDOTA in preparation of the heavy metal cadmium artificial antigen.
Owner:ZHEJIANG GONGSHANG UNIVERSITY
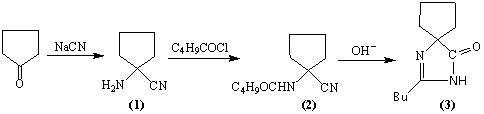
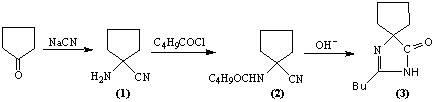
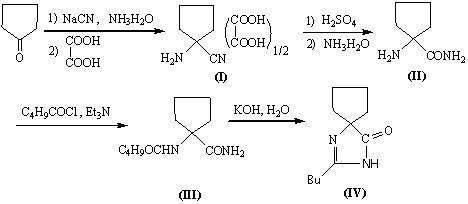
"One-pot" synthesis of irbesartan intermediate
InactiveCN102285923ASimple process routeEasy to operateOrganic chemistryCyanide compoundHypertension medications
The invention relates to an improved synthesis process of an antihypertensive drug irbesartan intermediate 2-butyl-1,3-diazaspiro[4.4]nonane-1-en-4-one. Cyclopentanone undergoes an addition reaction to obtain 1-aminocyclopentyl cyanide. The cyanide is first acylated, then hydrolyzed and cyclized under alkaline conditions to obtain the product. The positive effect of the present invention is that the first hydrolysis, then acylation and cyclization reported in the prior art is improved to first acylation, then hydrolysis and cyclization, each step does not go through the separation process, and the "one pot method" is realized Production. The operation process is simplified, the product yield is improved, the product quality is guaranteed, the production cost is reduced, and it is more conducive to large-scale industrial production.
Owner:河南华商药业有限公司
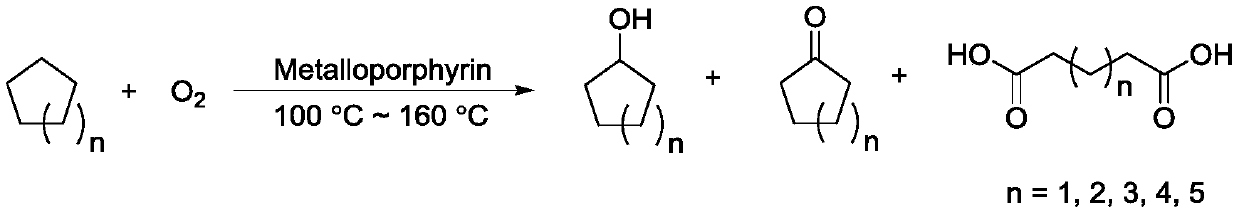
Cycloalkane catalytic oxidation method promoted by iron porphyrin
PendingCN110560169AReduce dosageHigh catalytic efficiencyPreparation by oxidation reactionsOrganic compound preparationAlkaneCyclononane
The invention relates to a cycloalkane catalytic oxidation method promoted by iron porphyrin. O2 is taken as an oxidant, Co (II) porphyrin and Mn (II) porphyrin are taken as main catalysts, and Fe (II) porphyrin is taken as a cocatalyst to carry out reacting at a temperature of 100-160 DEG C and pressure of 0.8-2.0MPa under a solvent-free condition to realize catalytic oxidation of cycloalkanes. The cycloalkanes mainly comprise cyclopentane, cyclohexane, cycloheptane, cyclooctane and cyclononane, and the corresponding oxidation products mainly comprise cycloalkyl alcohols, cycloalkyl ketones and alkyl diacids. The cycloalkane catalytic oxidation method has the advantages of high selectivity of cyclopentanone, cyclohexanol, cycloheptanone and cyclooctanone, small catalyst consumption and simple operation, and environmental friendliness is achieved by taking clean O2 as the oxidant. The disclosesd cycloalkane catalytic oxidation method promoted by iron porphyrin is a cycloalkane catalytic oxidation method with high product selectivity, simple operation and environmental friendliness.
Owner:ZHEJIANG UNIV OF TECH
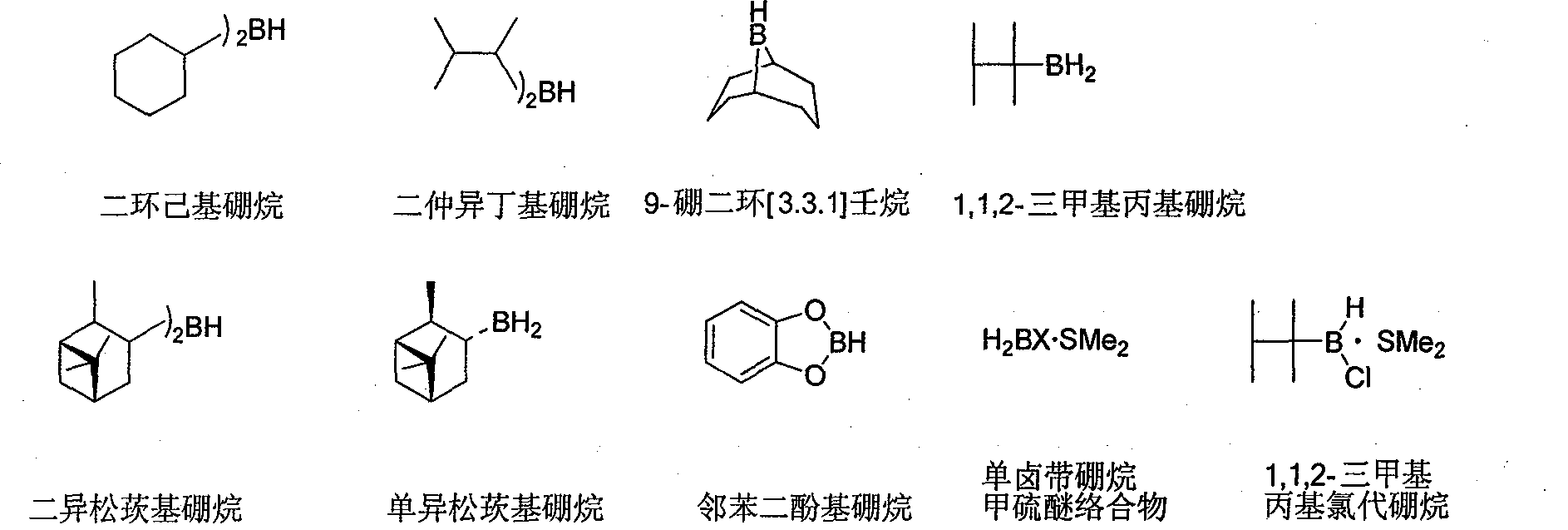
Preparation method of 9-boron bicyclo (3,3,1)-nonane (9-BBN)
InactiveCN103524541ASuppress generationHigh yieldGroup 3/13 element organic compoundsNonaneMolecular sieve
The invention provides a new synthesis method of 9-boron bicyclo (3,3,1)-nonane (9-BBN). 1,5-cyclooctadiene and borane are taken as raw materials, a reaction is carried out in a tetrahydrofuran solvent, and the 9-boron bicyclo (3,3,1)-nonane (9-BBN) is synthesized while yield is as high as 98%. The new synthesis method comprises the following steps of adding a smashed 4A molecular sieve, tetrahydrofuran, 1,5-cyclooctadiene and zirconium tetrachloride into a reaction flask, stirring for 1 hour, dropwise adding a dimethyl sulfide complex of borane at the temperature about 0 DEG C, carrying out a reflux reaction for 4 hours after dropwise addition is completed to obtain a product, cooling the obtained product to 0 DEG C and preserving heat for 3 hours, and filtering to obtain a 9-boron bicyclo (3,3,1)-nonane (9-BBN) solid product. The new synthesis method of the 9-boron bicyclo (3,3,1)-nonane (9-BBN) has the advantages of high yield, low cost, high product purity, easy operation, is beneficial to industrialization and has a good application prospect.
Owner:NANKAI UNIV
Degradable photoresist resin monomer synthesized from dimethyl bicyclo [3.3.1] nonane diketone and synthesis method of degradable photoresist resin monomer
PendingCN111606806AImprove corrosion resistanceIncreased etch resistanceOrganic compound preparationCarboxylic acid esters preparationAlkanePolymer science
The invention discloses a degradable photoresist resin monomer synthesized from dimethyl bicyclo [3.3. 1] nonane diketone and a synthesis method of the resin monomer. The structural formula of the resin monomer is shown in the specification, wherein R1 is a saturated alkane or a cycloalkane, and R2 is hydrogen or methyl. The synthesis method comprises the following steps: reacting (1R, 4R, 5S, 6S)-4, 6-dimethyl bicyclo [3.3. 1] nonane-2, 8-dione (I) with an alkyl Grignard reagent or a cycloalkyl Grignard reagent under the protection of inert gas to obtain an intermediate (II); reacting the intermediate (II) with acryloyl chloride or methacryloyl chloride under an alkaline condition to obtain a resin monomer (III); polymer resin formed by polymerizing the resin monomer provided by the invention and other resin monomers has better etching resistance, is beneficial to improving the edge roughness of a developed pattern, and greatly improves the resolution of a photoetching pattern.
Owner:XUZHOU B&C CHEM CO LTD
5-dichloroacetyl-3,6-dimethyl-3-ethyl-9-oxa-1,5-diazabicyclo[4.3.0]nonane and synthetic method
InactiveCN101381369AOvercome the shortcomings of serious pollutionHigh yieldBiocideOrganic chemistryNonaneOrganic synthesis
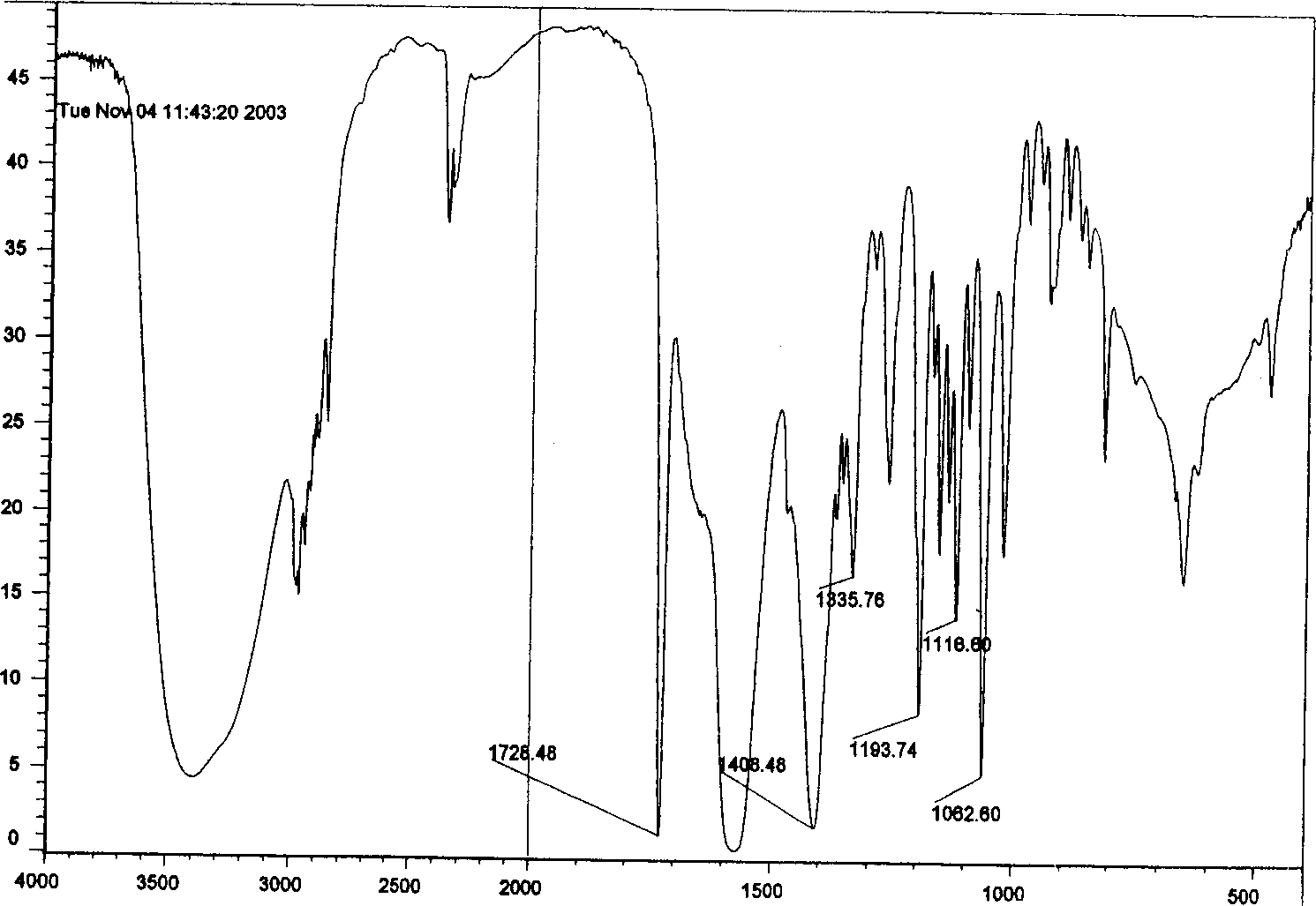
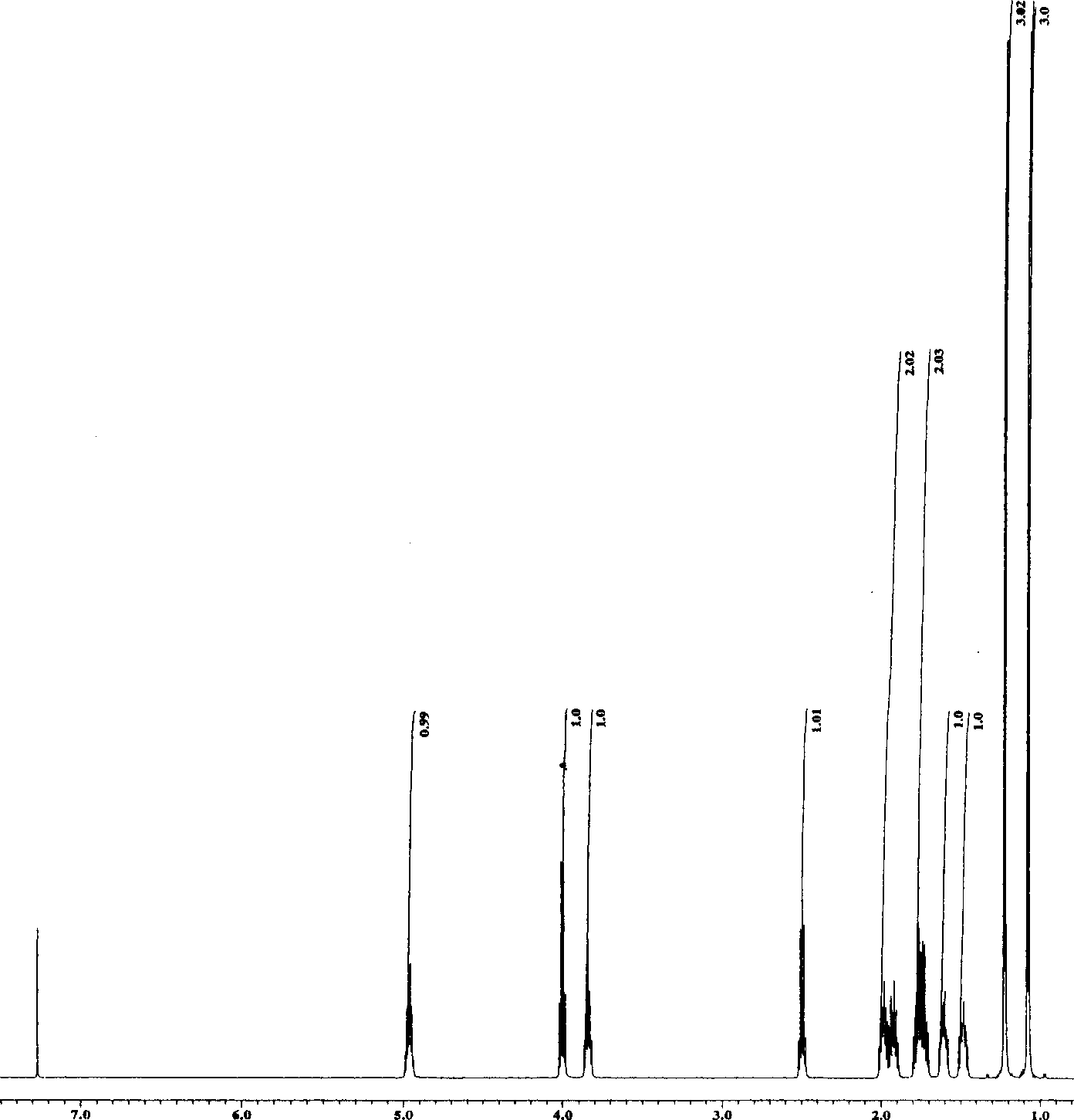
The invention relates to 5-dichloroacetyl-3, 6-dimethyl-3-ethyl-9-oxa-1, 5-diazabicylo (4.3.0) nonane and a synthesis method thereof, which belong to the organic synthesis technology. The structural formula of the 5-dichloroacetyl-3, 6-dimethyl-3-ethyl-9-oxa-1, 5-diazabicylo (4.3.0) nonane is as shown in a figure. The synthesis method is as follows: firstly, an acetylpropionic acid and 2-methyl-2-ethyl-1, 3-propylene diamine are taken as raw materials, and water in a system is separated in a solvent by the reflux water diversion method; secondly, the temperature of the system is reduced to 50 DEG C, and an acid-binding agent is added into the system to make the pH value of the system maintain 8.4; thirdly, the temperature of the system is controlled, dichloroacetyl chloride is dripped into the system to maintain constant pH value, and is stirred for a certain period; and fourthly, organic phases are washed into neutrality, and the product of the 5-dichloroacetyl-3, 6-dimethyl-3-ethyl-9-oxa-1, 5-diazabicylo (4.3.0) nonane is obtained after drying through anhydrous MgSO4, removal of a solvent and recrystallization of obtained coarse products by ethyl acetate-petroleum ether. The product and the method have the advantages of easily obtained raw materials, high yield, simple operation, short reaction period, low production cost and no environmental pollution.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Photoresist resin monomer synthesized from 3-ethylbicyclo[3.3.1]nonane-2,4-dione, and synthesis method thereof
PendingCN111138280AImprove corrosion resistanceImprove solubilityPreparation from carboxylic acid halidesPhotosensitive materials for photomechanical apparatusChemical synthesisAlkane
The invention discloses a photoresist resin monomer synthesized from 3-ethylbicyclo[3.3.1]nonane-2,4-dione, and a synthesis method thereof, and belongs to the technical fields of chemical synthesis and photoetching. The structural general formula of the photoresist resin monomer is represented by formula I shown in the description; and in the formula I, R1 is saturated alkane or cycloalkane, and R2 is hydrogen or a methyl group. The synthesis method comprises the following steps: carrying out a Grignard reaction on 3-ethylbicyclo[3.3.1] nonane-2,4-dione and an alkyl Grignard reagent or a cycloalkyl Grignard reagent under the protection of an inert gas, adding water for quenching after the Grignard reaction is finished, and carrying out post-treatment purification to obtain an intermediate;and carrying out an esterification reaction on the intermediate and acryloyl chloride or methacryloyl chloride, and carrying out post-treatment purification after the esterification reaction is finished in order to obtain the resin monomer. The resin monomer is a degradable resin monomer, and polymer resin containing the resin monomer has good etching resistance and can improve the resolution ofphotoresist photoetching patterns.
Owner:上海博栋化学科技有限公司
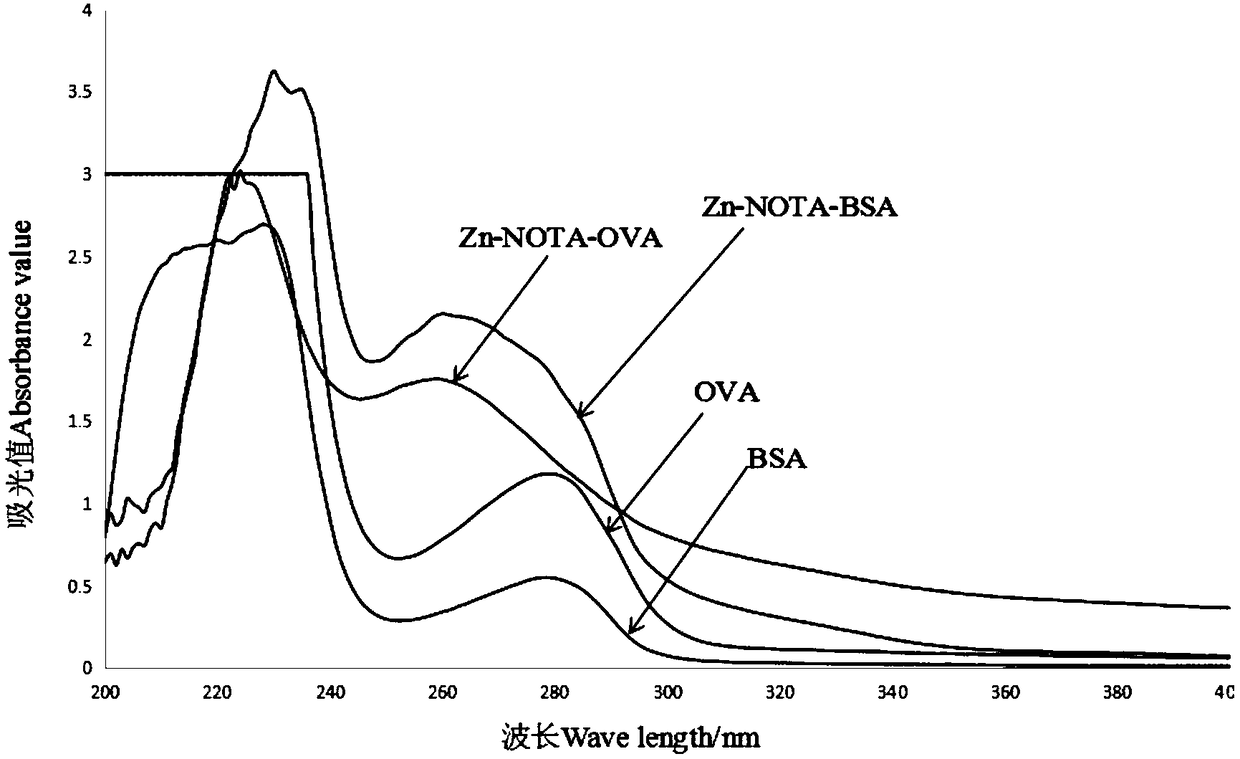
Preparation method of heavy metal zinc artificial antigen and application of NOTA in preparation of heavy metal zinc artificial antigen reagent
ActiveCN108148129AArtificial antigen stabilizationHigh titer of antiserumOvalbuminSerum albuminCyclononaneCarrier protein
The invention discloses a preparation method of heavy metal zinc artificial antigen, which applies 2-S-(4- aminobenzene)-1, 4, 7, 10 tetraazacyclo cyclononane-1, 4, 7, 10-acetate tetrahydrate(p-NH2-Bn-NOTA, NOTA for short) as cheating agent, and couples mercury ion chelating agent composite with carrier protein bovine serum albumin BSA or egg albumin OVA; thus the artificial antigen is prepared. The method adds the sodium borohydride processing step on the basis of traditional method in the past, and improves the coupling efficiency and antiserum titre. The invention further discloses an application of NOTA in preparation of the heavy metal zinc artificial antigen.
Owner:ZHEJIANG GONGSHANG UNIVERSITY
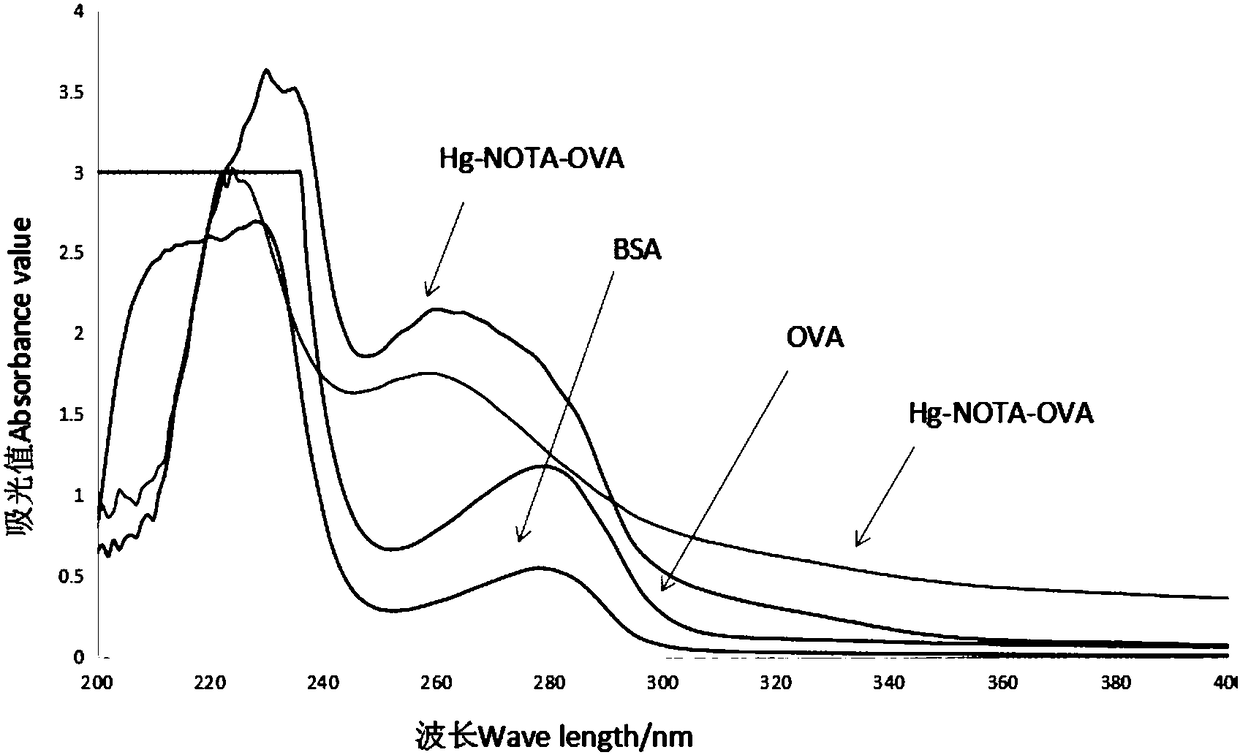
Preparation method of heavy metal mercury artificial antigen and application of NOTA in preparation of heavy metal mercury artificial antigen reagent
ActiveCN108148130AArtificial antigen stabilizationHigh titer of antiserumOvalbuminSerum albuminCyclononaneCarrier protein
The invention discloses a preparation method of heavy metal mercury artificial antigen, which applies 2-S-(4- aminobenzene)-1, 4, 7, 10 tetraazacyclo cyclononane-1, 4, 7, 10-acetate tetrahydrate(p-NH2-Bn-NOTA, NOTA for short) as cheating agent, and couples mercury ion chelating agent composite with carrier protein bovine serum albumin BSA or egg albumin OVA; thus the artificial antigen is prepared. The method adds the sodium borohydride processing step on the basis of traditional method in the past, and improves the coupling efficiency and antiserum titre. The invention further discloses an application of NOTA in preparation of the heavy metal mercury artificial antigen.
Owner:ZHEJIANG GONGSHANG UNIVERSITY
2-nitro-2-azaadamantane-4, 6, 8-triol trinitrate and preparation method thereof
PendingCN114195787ARaw materials are easy to getThe synthesis steps are simpleOrganic chemistryNitrated pentaerythritol explosive compositionsNonaneDiketone
The invention discloses 2-nitro-2-aza adamantane-4, 6, 8-triol trinitrate and a preparation method of the 2-nitro-2-aza adamantane-4, 6, 8-triol trinitrate. According to the method, 9-hydroxyl bicyclo [3.3. 1] nonane-2, 6-diketone is used as a raw material, the 2-nitro-2-azaadamantane-4, 6, 8-triol trinitrate is finally synthesized through the steps of hydrazone formation, elimination, epoxidation, cyclization, acetylation, nitration and the like, the compound has higher density and energy, the synthesis method is simple, the yield is high, and the performance is stable.
Owner:NANJING UNIV OF SCI & TECH
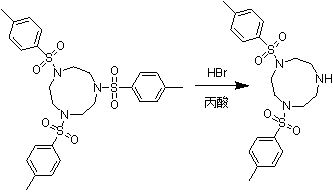
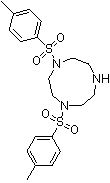
Method for preparing moxifloxacin intermediate (S, S)-2, 8-diazabicyclo [4, 3, 0] nonane
The invention provides a method for preparing a moxifloxacin intermediate (S, S)-2, 8-diazabicyclo [4, 3, 0] nonane, and relates to the technical field of organic synthesis. According to the present invention, the 2-chloromethyl methyl nicotinate is subjected to asymmetric hydrogenation catalysis so as to obtain the intermediate with high chiral purity, chiral resolution is not required, and the moxifloxacin intermediate (S, S)-2, 8-diazabicyclo [4, 3, 0] nonane can be obtained through ammonolysis, reduction and cyclization. According to the method provided by the invention, chiral resolution is not needed, the process is simple, the process steps are short, the cost is low, and the product is high in chiral purity and high in total yield.
Owner:TAIZHOU BIOMEDICAL & CHEM IND RES INST CO LTD
Method for preparing moxifloxacin intermediate (S, S)-2, 8-diazabicyclo [4, 3, 0] nonane
The invention provides a method for preparing a moxifloxacin intermediate (S, S)-2, 8-diazabicyclo [4, 3, 0] nonane, and relates to the technical field of organic synthesis. According to the method, the azaphthalide is used as a raw material, and due to the ring structure of the azaphthalide, the other two non-corresponding chiral isomers which are not needed in chiral reduction are almost not generated; the chiral purity of the intermediate obtained through reduction is very high, resolution is not needed, and (S, S)-2, 8-diazabicyclo [4, 3, 0] nonane can be obtained directly through ammonolysis, reduction, chlorination and cyclization subsequently. According to the method provided by the invention, chiral resolution is not needed, the process is simple, the process steps are short, the cost is low, and the product is high in chiral purity and high in total yield. Furthermore, in the subsequent product salifying and refining step, carboxylic acid with a chiral structure does not need to be used for salifying, and common achiral carboxylic acid can be used for refining, so the product purity is further improved.
Owner:TAIZHOU BIOMEDICAL & CHEM IND RES INST CO LTD
A method for preparing moxifloxacin intermediate (s,s)-2,8-diazabicyclo[4,3,0]nonane
The invention provides a method for preparing a moxifloxacin intermediate (S,S)-2,8-diazabicyclo[4,3,0]nonane, and relates to the technical field of organic synthesis. The present invention uses azaphthalide as a raw material. Due to the cyclic structure of azaphthalide itself, the other two non-responsive chiral isomers that are not needed in chiral reduction are hardly produced, and the reduced intermediate itself The chiral purity is very high, no further resolution is required, and (S,S)‑2,8‑diazabicyclo[4,3,0 ] Nonane. The method provided by the invention does not need chiral resolution, has simple process, short process steps, low cost, high chiral purity of products and high total yield. Furthermore, in the subsequent steps of product salification and refining, there is no need to use carboxylic acids containing chiral structures to form salts, and ordinary achiral carboxylic acids can be used for refining to further improve product purity.
Owner:TAIZHOU BIOMEDICAL & CHEM IND RES INST CO LTD
A kind of preparation method of pulp, fabric bleaching catalyst ligand
ActiveCN103360332BEasy to prepareRaw materials are easy to getOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystCyclononane
The invention discloses a method for preparing a ligand of a pulp and fabric low-temperature bleaching catalyst. The main steps include: dissolving diethylenetriamine with a sulfonyl group in an organic solvent, adding an alkaline reagent and ethylene glycol dicarboxylic acid ester, react at 80-150°C for 6-20 hours, after the reaction, cool to room temperature and filter to obtain 1,4,7-trisulfonyl-1,4,7-triazacyclononane; Add the solid to sulfuric acid, stir at 80-130°C for 2-12 hours, add alkali to neutralize after cooling down, then add formaldehyde and formic acid to reflux to obtain 1,4,7-trimethyl-1,4,7-triaza Cyclononane. The preparation method of the invention is simple, the raw materials are easy to obtain, and the three wastes are less, which is suitable for industrial production and meets the environmental protection requirements of the current industrial production.
Owner:苏州正济药业有限公司
Compound of substituted nonane oxide-2-ketone, preparation method and application
A substituted oxyheteroarylnonane-2-one compound used for preparing cell cycle depressant, cell wither inducer and antineoplastic medicine isprepared from streptomyces flavorectus.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
H-GI-POF high-temperature optical fiber and preparation method thereof
PendingCN112213818AShort transmission delayImprove signal transmission bandwidthOptical fibre with multilayer core/claddingConjugated synthetic polymer artificial filamentsFiberCyclononane
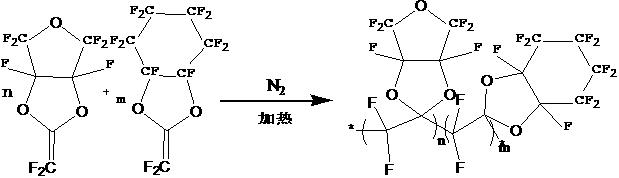
The invention belongs to the technical field of optical fibers, and particularly relates to an h-GI-POF high-temperature optical fiber and a preparation method thereof. The h-GI-POF high-temperature light-guide fiber comprises an inner core layer and an outer skin layer, the inner core layer and the outer skin layer are arranged in a concentric structure, the inner core layer and the outer skin layer are composed of high polymers with different refractive indexes, the high polymers are formed by copolymerization of perfluorodioxolane and perfluoro dioxin bicyclononane, and the refractive indexof the inner core layer is larger than that of the outer skin layer. The inner core layer is integrally formed by five core layers of which the refractive indexes are gradually reduced from the coreto the outside, and the outer skin layer is formed by a single-layer skin layer, so that the transparency reaches 98% or above, the glass transition temperature Tg exceeds 150 DEG C, and the high-temperature optical fiber is suitable for ultra-high-speed communication. Compared with the SI-POF in the prior art, six layers of core materials are used, and each core layer has a gradual change refractive index, so that the incident light transmission delay is shorter than that of the common SI-POF, the signal transmission bandwidth is larger, and the transmission speed is higher.
Owner:中闽光纤科技有限公司
Adhesive resin and preparation method and applications thereof
InactiveCN106905891AStrong weather resistanceStrong water resistanceNon-macromolecular adhesive additivesGraft polymer adhesivesLow-density polyethylenePolymer science
The invention provides an adhesive resin, which comprises high-density polyethylene, ethylene-vinyl acetate copolymer, low-density polyethylene, 1,1-bis-(t-butylperoxy)-3,3,5-trimethyl cyclohexane, 3,3,6,6,9,9-Hexamethyl-1,2,4,5-teraoxacyclononane, 2,5-dimethyl-2,5-bis (benzoyl peroxide) hexane and maleic anhydride. The maleic anhydride is grafted by adopting active grafting technology, and polarity function groups are grafted on polyethylene molecular chains. Compared with the prior art, the adhesive resin has the characteristics of being high in adhesion intensity, strong in adhesion durability, and strong in weather resistance and water resistance performance, and can be used for a glass filament winding reinforced polyethylene composite tube.
Owner:SHANGHAI BANZAN MACROMOLECULE MATERIAL
Synthesis method of 3,7-dinitro-1,3,5,7-4-azabicyclo[3,3,1]nonane
Owner:XIAN MODERN CHEM RES INST
1,2-Dioxane[3,4-f]Nitrooxycyclononane Derivatives and Their Synthesis and Application
ActiveCN111018885BGood anti-inflammatory activityNovel structureOrganic active ingredientsOrganic chemistryPtru catalystPhotosensitizer
The invention discloses a series of 1,2-dioxcyclohexene [3,4-f]nitrogenated cyclononane derivatives, their synthesis method and application. The 1,2-dioxane[3,4-f]nitroxycyclononane derivative of the present invention has the structure shown in the following formula (I), and its synthesis method mainly includes the following steps: take the following formula The compound shown in (II), the compound shown in formula (III), the photosensitizer and the catalyst are placed in an organic solvent, and react under light irradiation in the presence of oxygen to obtain the crude product of the target compound. The method of the invention is simple and easy to control, and some target compounds obtained have good anti-inflammatory activity and certain potential medicinal value. The compounds of structures shown in the formula (I), formula (II) and formula (III) are respectively as follows:
Owner:南京搏克思新材料科技有限公司
Preparation of racemic cis 8-benzyl-2,8-diazabicyclo[4,3,0]nonane
ActiveCN112110916BHigh selectivityReduce dosageOrganic chemistryCatalyst protectionNonanePtru catalyst
The invention provides a preparation process for racemic cis-8-benzyl-2,8-diazabicyclo[4,3,0]nonane, which comprises the following steps: Step S1: preparing an attachment carrier: immersing a solid carrier into Prepare an attachment carrier in a roughening solution, the roughening solution is a sodium hydroxide solution or a sodium bifluoride solution; Step S2: Prepare a platinum dehydrogenation catalyst: use an impregnation method to support the precious metal platinum by impregnating it into an impregnation solution containing noble metal ions On the solid carrier, in parts by weight, the impregnation solution includes 30-40 parts of chloroplatinic acid and 1-5 parts of rice furoic acid; step S3: piperidine ring dehydrogenation reaction; step S4: high-pressure hydrogenation reaction. The invention provides a method for preparing racemic cis-8-benzyl-2,8-diazabicyclo[4,3,0]nonane with high selectivity, high catalytic efficiency and high product purity.
Owner:SHAYANG QINJIANG CHEM
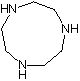
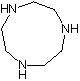
Synthesis method of 1, 4, 7-triazacyclononane
The invention provides a synthesis method of 1, 4, 7-triazacyclononane. The preparation method comprises the step of: carrying out condensation ring-closure reaction on N, N'-bis (2-chloroethyl) ethylenediamine hydrochloride and ethylenediamine at low temperature to obtain 1, 4, 7-triazacyclononane. According to the method, the reaction temperature ranges from -50 DEG C to -30 DEG C, and the molar ratio of N, N'-bis (2-chloroethyl) ethylenediamine hydrochloride to ethylenediamine is 1: (0.95-1). 4-dimethylaminopyridine (DMAP) is adopted as an acid-binding agent, and the molar ratio of the DMAP to the N, N'-bis (2-chloroethyl) ethylenediamine hydrochloride is (3.9-4.1): 1. Carbon tetrachloride is selected as a reaction solvent, and the mass ratio of the carbon tetrachloride to the N, N'-bis (2-chloroethyl) ethylenediamine hydrochloride is 2: 1. According to the process, other side reactions can be effectively avoided, the highest reaction yield can reach 78% or above, and the product purity can reach 94% or above.
Owner:仪征市海帆化工有限公司
Preparation method of 1,6-dioxaspiro [4, 4] nonane-3,8-diene derivative
The invention discloses a reparation method of a 1,6-dioxaspiro [4, 4] nonane-3,8-diene derivative. The preparation method comprises the following steps: 1) adding isonitrile, acetylenic esters and carbon dioxide in a 25 ml round-bottom flask, and stirring vigorously under certain conditions; and 2) after the reaction, conducting reduced pressure distillation to remove the solvent and conducting column chromatography isolation by using petroleum ether-acetone as an eluent to obtain a product. The invention finds for the first time a multicomponent compound with two carbon oxygen double bonds of carbon dioxide participating at the same time; and the method has the advantages of simple reaction operations, convenient posttreatment, mild conditions and operation conditions of atmospheric pressure and no catalysis.
Owner:苏州楚凯药业有限公司 +1
A kind of preparation method of macrolide precursor
ActiveCN109096240BSimple processSubstrate structure is simple and easy to obtainOrganic chemistryCyclohexanoneNonane
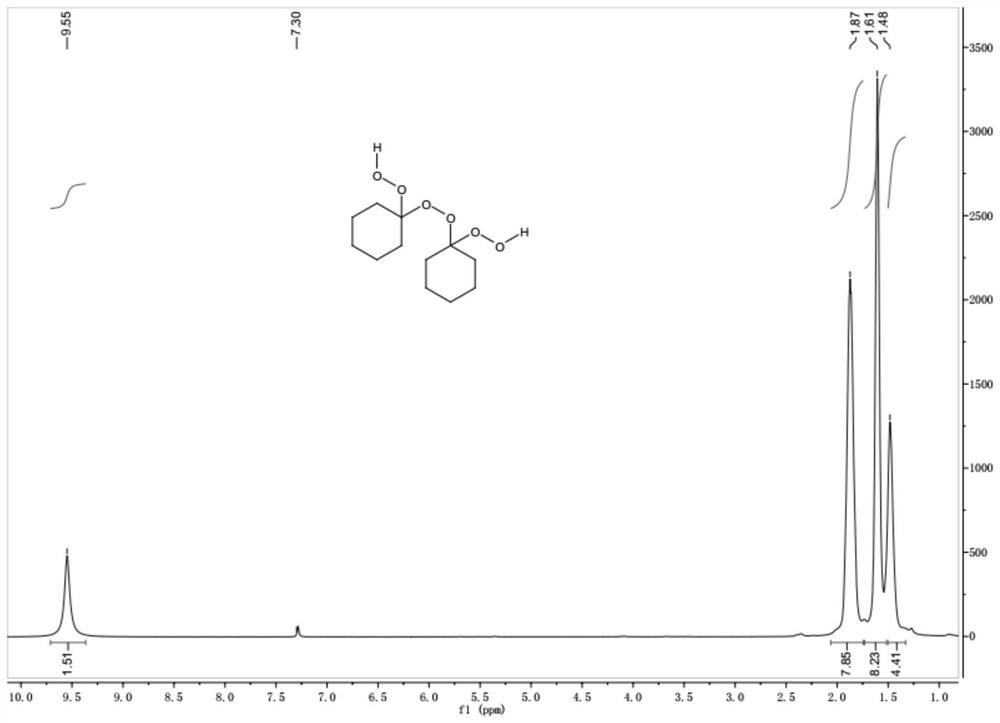
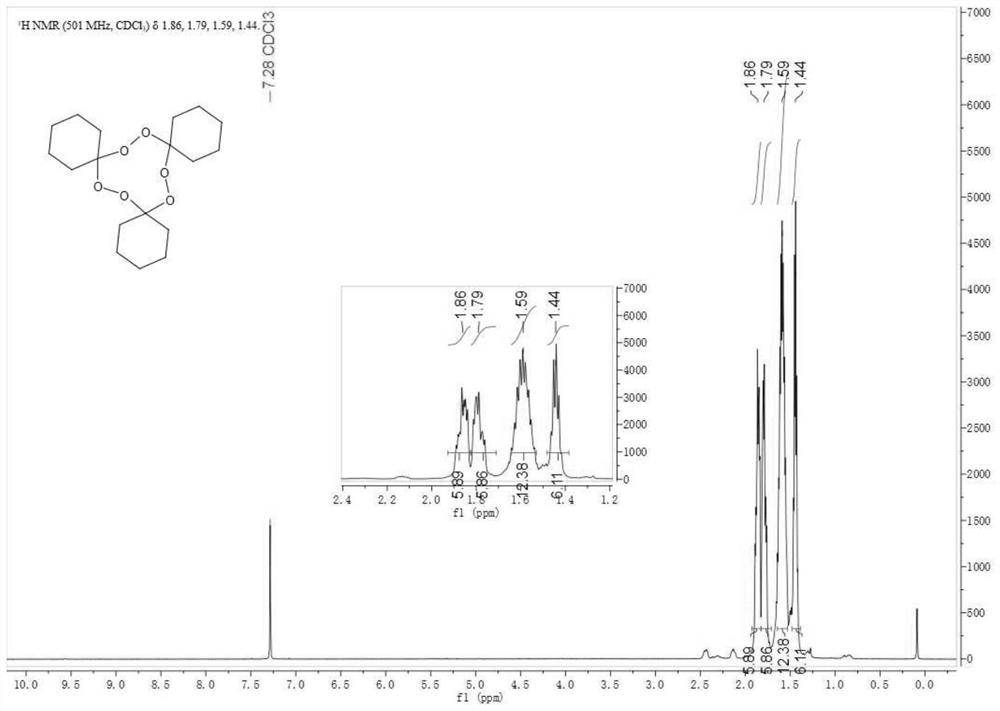
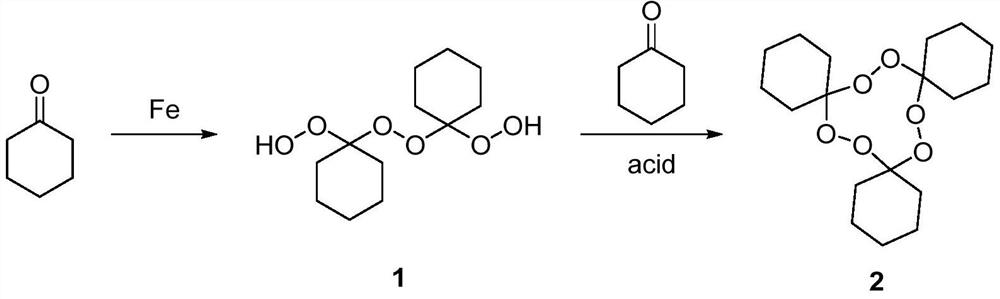
The invention belongs to the technical field of organic synthesis and particularly discloses a method for preparing a macrolide precursor. The method comprises the following steps: (1) mixing cyclohexanone and hydrogen peroxide, further adding a ferric salt catalyst, carrying out a reaction for 1-8 hours at the room temperature, and carrying out column chromatography separation after the reactionis completed so as to obtain a main product 1,1'-dihydroperoxide dicyclohexyl peroxide; (2) condensing the main product with cyclohexanone under catalysis of an organic acid, and after the reaction ends, carrying out separation and purification, thereby obtaining the macrolide precursor 1,2,4,5,7,8-hexaoxa-3,6,9-tricyclohexylidene cyclononane. The cyclohexanone which is low in price and easy to obtain is adopted as a raw material, and the product is prepared through two steps of reactions under catalysis of a ferric salt and a small amount of the organic acid at 20-45 DEG C; the method is simple and convenient in process, high in yield, applicable to popularization and application, and the product prepared by using the method can be applied to fields such as a cigarette fixative agent.
Owner:SHANGHAI INST OF TECH
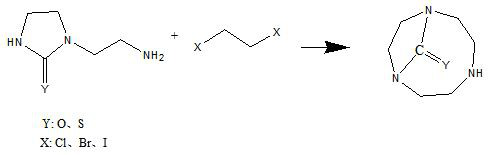
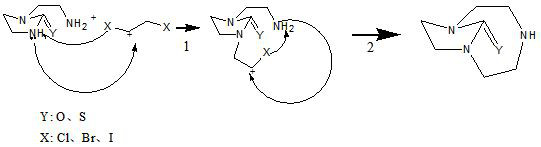
Synthetic method of 1,4,7-triazacyclononane-1,4-one and 1,4,7-triazacyclononane-1,4-thione
The invention proposes a synthesis method of 1,4,7-triazacyclononane-1,4-ketone and 1,4,7-triazacyclononane-1,4-thione. 1,4,7‑Triazacyclononane‑1,4‑one and 1,4,7‑Triazacyclononane‑1,4‑thione are new compounds that can be used as 1,4,7‑ An important synthetic intermediate of triazacyclononane and its derivatives. In the present invention, 1-(2-aminoethyl)-2-imidazolinone compounds are condensed with 1,2-dihalogenated ethane compounds to obtain products, namely 1,4,7-triazacyclononane- 1,4-ketone and 1,4,7-triazacyclononane-1,4-thione. The process of the invention has the advantages of low raw material cost, high conversion rate, mild reaction conditions, simple and easy-to-control reaction process, high purity of the final product, and the like.
Owner:扬州市荣晶新材料科技有限公司
A method for synthesizing 5-fluoro-1-azabicyclo[3,2,2]nonane and derivatives thereof
ActiveCN111732588BMild reaction conditionsAchieve synthesisOrganic chemistryChemical synthesisChromatographic separation
The invention relates to the technical field of organic chemical synthesis, and relates to a method for synthesizing 5-fluoro-1-azabicyclo[3, 2, 2]nonane and derivatives thereof. The preparation method comprises the following steps: reacting a 4-(hydroxymethyl)quinuclidine derivative with a fluorinating reagent at normal temperature, rearranging one six-membered ring of a quinine ring to obtain afluorine substituted product of a seven-membered ring, carrying out extraction, solvent removal and concentration on a reaction mixture, and carrying out column chromatography separation or sublimation to obtain pure 5-fluoro-1-azabicyclo[3, 2, 2]nonane and derivatives thereof. The 5-fluoro-1-azabicyclo[3, 2, 2]nonane and the derivative thereof are obtained through one-step synthesis by reacting the 4-(hydroxymethyl)quinuclidine derivative with the fluorinating reagent, and the method is discovered for the first time. The synthesis method is simple, has few synthesis steps, is easy to separate, and can realize large-scale synthesis of target products.
Owner:NANCHANG UNIV
Method for synthesizing 5-fluoro-1-azabicyclo[3, 2, 2]nonane and derivatives thereof
ActiveCN111732588AMild reaction conditionsAchieve synthesisOrganic chemistryChemical synthesisChromatographic separation
The invention relates to the technical field of organic chemical synthesis, and relates to a method for synthesizing 5-fluoro-1-azabicyclo[3, 2, 2]nonane and derivatives thereof. The preparation method comprises the following steps: reacting a 4-(hydroxymethyl)quinuclidine derivative with a fluorinating reagent at normal temperature, rearranging one six-membered ring of a quinine ring to obtain afluorine substituted product of a seven-membered ring, carrying out extraction, solvent removal and concentration on a reaction mixture, and carrying out column chromatography separation or sublimation to obtain pure 5-fluoro-1-azabicyclo[3, 2, 2]nonane and derivatives thereof. The 5-fluoro-1-azabicyclo[3, 2, 2]nonane and the derivative thereof are obtained through one-step synthesis by reacting the 4-(hydroxymethyl)quinuclidine derivative with the fluorinating reagent, and the method is discovered for the first time. The synthesis method is simple, has few synthesis steps, is easy to separate, and can realize large-scale synthesis of target products.
Owner:NANCHANG UNIV
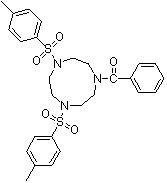
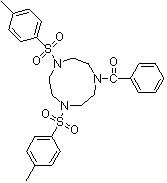
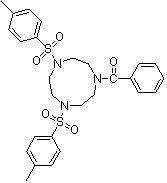
Synthesis method of 1,4-bis(p-toluenesulfonyl)-7-benzoyl trinitrocyclononane
The invention discloses a synthesis method of 1,4-bis(p-toluenesulfonyl)-7-benzoyl trinitrocyclononane, which comprises the following steps of: By taking N-benzoyl-O,O-p-toluenesulfonyl-diethanol amine and N,N'-bis(p-toluenesulfonyl)ethylenediamine as substrates and anhydrous potassium carbonate or anhydrous sodium carbonate as an acid-binding agent, carrying out two-phase reaction in a two-phase solvent of toluene or xylene and water to obtain the 1,4-bis(p-toluenesulfonyl)-7-benzoyl triazacyclononane. The molar ratio of the N-benzoyl-O,O-p-toluenesulfonyl-diethanol amine to the N,N'-di(p-toluenesulfonyl)ethylenediamine is 1:(1-1.05), the molar ratio of the acid-binding agent to the N-benzoyl-O,O-p-toluenesulfonyl-diethanol amine is (3-5):1, the reaction temperature is 90-100 DEG C, and the reaction time is 5-20 hours. The highest reaction yield can reach 85% or above, the reaction product can reach 96% or above without refining, and the synthesis requirements of various organic ligands are completely met. The process provided by the invention improves the intrinsic safety of the technological process, reduces the production and management cost, and has the advantages of mild reaction conditions and low production cost.
Owner:仪征市海帆化工有限公司
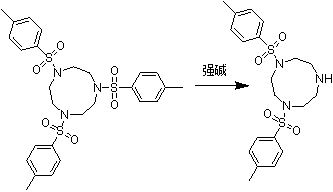
Synthesis method of 1,4-bis(p-toluenesulfonyl)trinitrocyclononane
PendingCN113277990AMeet the synthesis requirementsReduce typesOrganic chemistryBulk chemical productionOrganic acidPhenacyl
The invention discloses a synthesis method of 1,4-bis(p-toluenesulfonyl)trinitrocyclononane. The synthesis method comprises the following steps: with 1,4-bis(p-toluenesulfonyl)-7-benzoyltrinitrocyclononane as a substrate, removing a protecting group of a benzoyl group under an alkaline condition, and conducting a reaction under the condition that two p-toluenesulfonyl groups do not participate in the reaction so as to obtain the 1,4-bis(p-toluenesulfonyl)trinitrocyclononane. Reaction conditions are that a reflux reaction is adopted and reaction time is 10-36 hours. Compared with the prior art, the synthesis method of the invention has the following beneficial effects: the use of a large amount of inorganic acid and organic acid is avoided, and the problems of safety, environmental protection and the like are effectively guaranteed essentially; and reaction yield can be up to 90% or above.
Owner:仪征市海帆化工有限公司
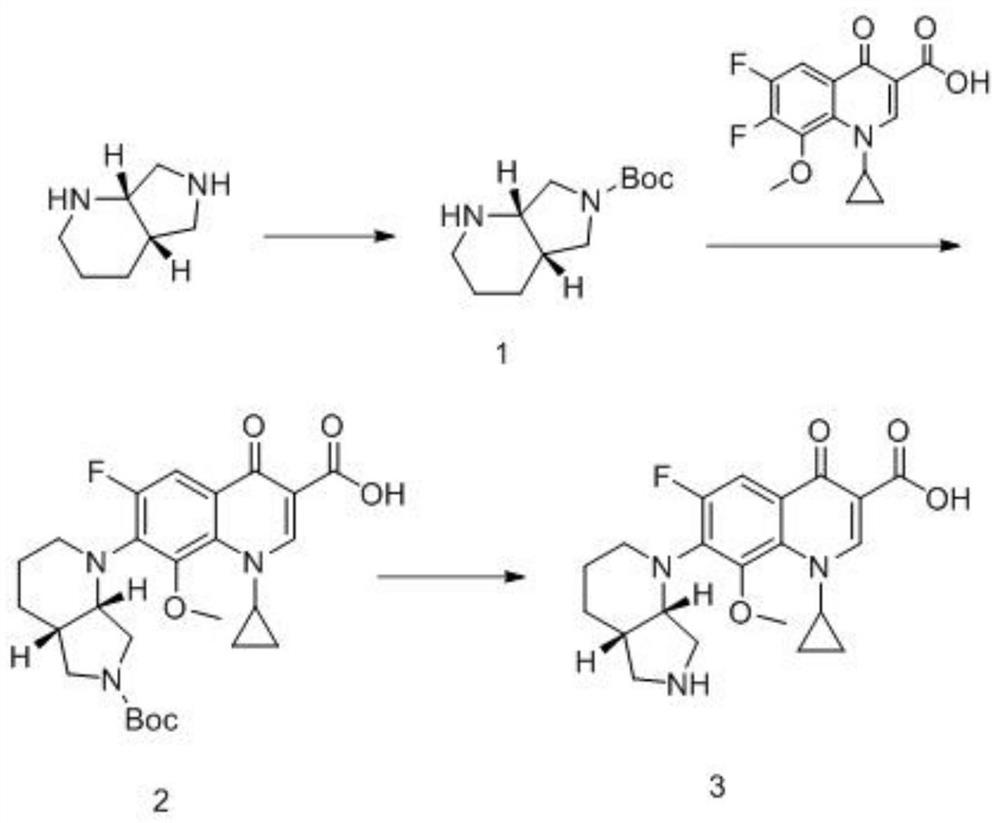
Quinolone analogue as well as preparation method and application thereof
PendingCN114105973ASimple manufacturing methodQuality improvementOrganic chemistryComponent separationNonaneCyclononane
The invention discloses a quinolone analogue and a preparation method and application thereof.The preparation method comprises the steps that S, S-2, 8-diazo-bicyclo [4.3. 0] nonane is dissolved in an organic solvent, and organic alkali is added; dissolving di-tert-butyl dicarbonate in an organic solvent, and dropwise adding into the reaction system to react to obtain a compound 1; dissolving the compound 1, slowly adding sodium hydride at 0-10 DEG C, and uniformly dispersing; the preparation method comprises the following steps: adding a compound 1 into a reaction system, dropwise adding a 1-cyclopropyl-6, 7-difluoro-8-methoxy-1, 4-dihydro-4-oxo-3-quinoline carboxylic acid solution into the reaction system, and reacting to obtain a compound 2; and dissolving the compound 2, and adding acid for reaction to obtain a compound 3, namely the target quinolone analogue. The preparation method of the quinolone analogue is simple and easy to implement, and the quinolone analogue can be prepared on a large scale. When the quinolone analogue is used for detecting related substances of moxifloxacin hydrochloride and preparations thereof, the quality of moxifloxacin hydrochloride can be further improved, the safety is improved, and the risk of medication is reduced.
Owner:CHANGZHOU FANGYUAN PHARMA +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


![Degradable photoresist resin monomer synthesized from dimethyl bicyclo [3.3.1] nonane diketone and synthesis method of degradable photoresist resin monomer Degradable photoresist resin monomer synthesized from dimethyl bicyclo [3.3.1] nonane diketone and synthesis method of degradable photoresist resin monomer](https://images-eureka.patsnap.com/patent_img/10776607-1706-443f-a502-9c4c1b2ff092/FDA0002544917710000012.png)
![Degradable photoresist resin monomer synthesized from dimethyl bicyclo [3.3.1] nonane diketone and synthesis method of degradable photoresist resin monomer Degradable photoresist resin monomer synthesized from dimethyl bicyclo [3.3.1] nonane diketone and synthesis method of degradable photoresist resin monomer](https://images-eureka.patsnap.com/patent_img/10776607-1706-443f-a502-9c4c1b2ff092/FDA0002544917710000013.png)
![Degradable photoresist resin monomer synthesized from dimethyl bicyclo [3.3.1] nonane diketone and synthesis method of degradable photoresist resin monomer Degradable photoresist resin monomer synthesized from dimethyl bicyclo [3.3.1] nonane diketone and synthesis method of degradable photoresist resin monomer](https://images-eureka.patsnap.com/patent_img/10776607-1706-443f-a502-9c4c1b2ff092/BDA0002544917720000021.png)
![5-dichloroacetyl-3,6-dimethyl-3-ethyl-9-oxa-1,5-diazabicyclo[4.3.0]nonane and synthetic method 5-dichloroacetyl-3,6-dimethyl-3-ethyl-9-oxa-1,5-diazabicyclo[4.3.0]nonane and synthetic method](https://images-eureka.patsnap.com/patent_img/8db8a398-af65-43ef-821f-fd6175232445/a200810137362c00021.PNG)
![5-dichloroacetyl-3,6-dimethyl-3-ethyl-9-oxa-1,5-diazabicyclo[4.3.0]nonane and synthetic method 5-dichloroacetyl-3,6-dimethyl-3-ethyl-9-oxa-1,5-diazabicyclo[4.3.0]nonane and synthetic method](https://images-eureka.patsnap.com/patent_img/8db8a398-af65-43ef-821f-fd6175232445/a200810137362d00031.PNG)
![5-dichloroacetyl-3,6-dimethyl-3-ethyl-9-oxa-1,5-diazabicyclo[4.3.0]nonane and synthetic method 5-dichloroacetyl-3,6-dimethyl-3-ethyl-9-oxa-1,5-diazabicyclo[4.3.0]nonane and synthetic method](https://images-eureka.patsnap.com/patent_img/8db8a398-af65-43ef-821f-fd6175232445/a200810137362d00051.PNG)
![Photoresist resin monomer synthesized from 3-ethylbicyclo[3.3.1]nonane-2,4-dione, and synthesis method thereof Photoresist resin monomer synthesized from 3-ethylbicyclo[3.3.1]nonane-2,4-dione, and synthesis method thereof](https://images-eureka.patsnap.com/patent_img/1b93c67d-b4f3-486e-b83e-3b15890532a6/FDA0002333947290000011.png)
![Photoresist resin monomer synthesized from 3-ethylbicyclo[3.3.1]nonane-2,4-dione, and synthesis method thereof Photoresist resin monomer synthesized from 3-ethylbicyclo[3.3.1]nonane-2,4-dione, and synthesis method thereof](https://images-eureka.patsnap.com/patent_img/1b93c67d-b4f3-486e-b83e-3b15890532a6/FDA0002333947290000012.png)
![Photoresist resin monomer synthesized from 3-ethylbicyclo[3.3.1]nonane-2,4-dione, and synthesis method thereof Photoresist resin monomer synthesized from 3-ethylbicyclo[3.3.1]nonane-2,4-dione, and synthesis method thereof](https://images-eureka.patsnap.com/patent_img/1b93c67d-b4f3-486e-b83e-3b15890532a6/BDA0002333947300000021.png)


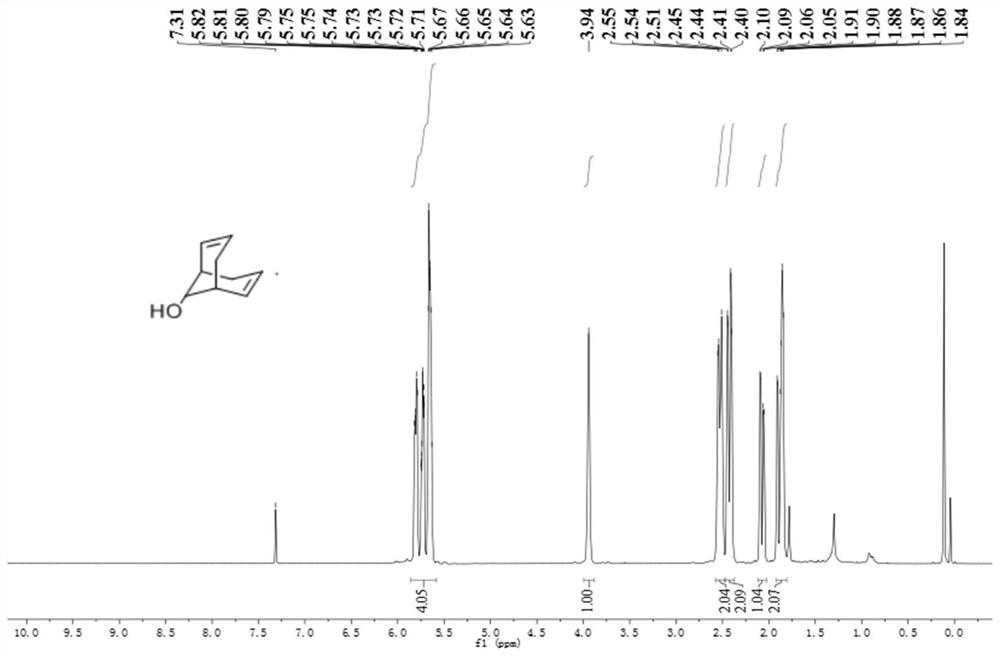
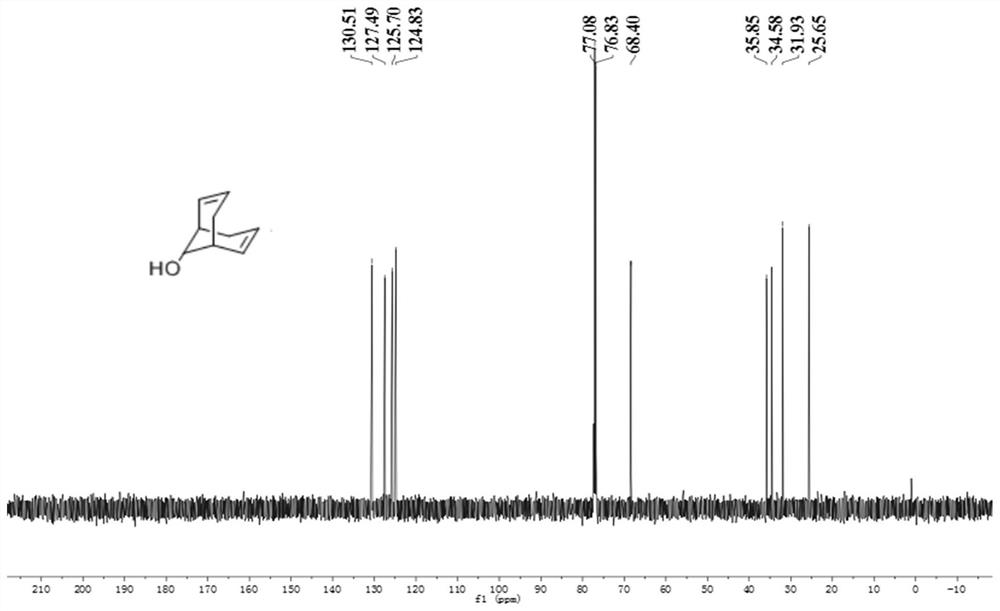
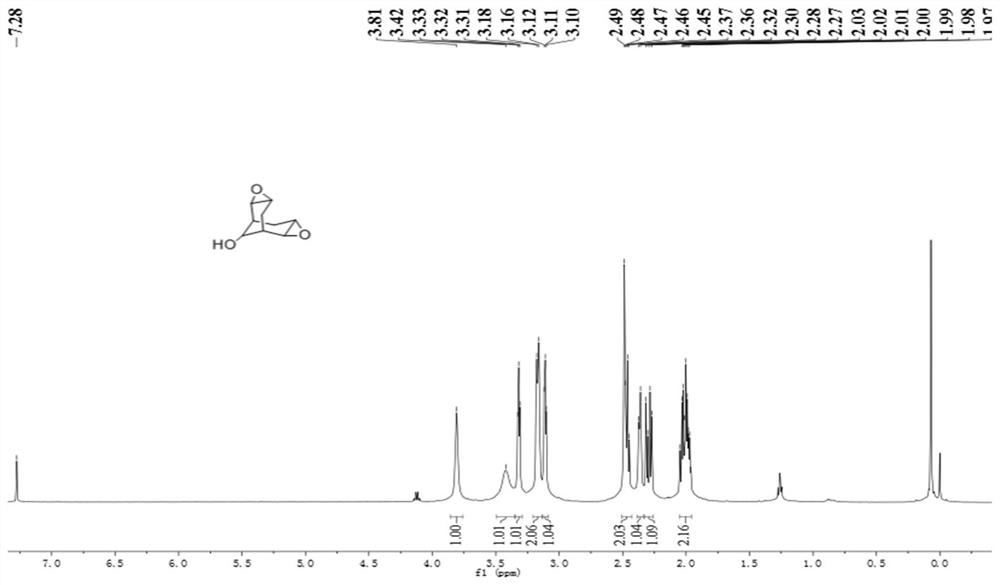
![Method for preparing moxifloxacin intermediate (S, S)-2, 8-diazabicyclo [4, 3, 0] nonane Method for preparing moxifloxacin intermediate (S, S)-2, 8-diazabicyclo [4, 3, 0] nonane](https://images-eureka.patsnap.com/patent_img/957da25f-1160-4b22-81d6-01f9aaa929a8/HDA0002937570980000011.png)
![Method for preparing moxifloxacin intermediate (S, S)-2, 8-diazabicyclo [4, 3, 0] nonane Method for preparing moxifloxacin intermediate (S, S)-2, 8-diazabicyclo [4, 3, 0] nonane](https://images-eureka.patsnap.com/patent_img/957da25f-1160-4b22-81d6-01f9aaa929a8/BDA0002937570970000051.png)
![Method for preparing moxifloxacin intermediate (S, S)-2, 8-diazabicyclo [4, 3, 0] nonane Method for preparing moxifloxacin intermediate (S, S)-2, 8-diazabicyclo [4, 3, 0] nonane](https://images-eureka.patsnap.com/patent_img/957da25f-1160-4b22-81d6-01f9aaa929a8/BDA0002937570970000061.png)
![Method for preparing moxifloxacin intermediate (S, S)-2, 8-diazabicyclo [4, 3, 0] nonane Method for preparing moxifloxacin intermediate (S, S)-2, 8-diazabicyclo [4, 3, 0] nonane](https://images-eureka.patsnap.com/patent_img/f4a96a8b-1fc6-4890-8c89-f72d04e2bc55/HDA0002937573050000011.png)
![Method for preparing moxifloxacin intermediate (S, S)-2, 8-diazabicyclo [4, 3, 0] nonane Method for preparing moxifloxacin intermediate (S, S)-2, 8-diazabicyclo [4, 3, 0] nonane](https://images-eureka.patsnap.com/patent_img/f4a96a8b-1fc6-4890-8c89-f72d04e2bc55/BDA0002937573040000051.png)
![Method for preparing moxifloxacin intermediate (S, S)-2, 8-diazabicyclo [4, 3, 0] nonane Method for preparing moxifloxacin intermediate (S, S)-2, 8-diazabicyclo [4, 3, 0] nonane](https://images-eureka.patsnap.com/patent_img/f4a96a8b-1fc6-4890-8c89-f72d04e2bc55/BDA0002937573040000061.png)
![A method for preparing moxifloxacin intermediate (s,s)-2,8-diazabicyclo[4,3,0]nonane A method for preparing moxifloxacin intermediate (s,s)-2,8-diazabicyclo[4,3,0]nonane](https://images-eureka.patsnap.com/patent_img/0a515ebf-9322-4ac1-8a78-5728eadb6705/HDA0002937573050000011.png)
![A method for preparing moxifloxacin intermediate (s,s)-2,8-diazabicyclo[4,3,0]nonane A method for preparing moxifloxacin intermediate (s,s)-2,8-diazabicyclo[4,3,0]nonane](https://images-eureka.patsnap.com/patent_img/0a515ebf-9322-4ac1-8a78-5728eadb6705/BDA0002937573040000051.png)
![A method for preparing moxifloxacin intermediate (s,s)-2,8-diazabicyclo[4,3,0]nonane A method for preparing moxifloxacin intermediate (s,s)-2,8-diazabicyclo[4,3,0]nonane](https://images-eureka.patsnap.com/patent_img/0a515ebf-9322-4ac1-8a78-5728eadb6705/BDA0002937573040000061.png)
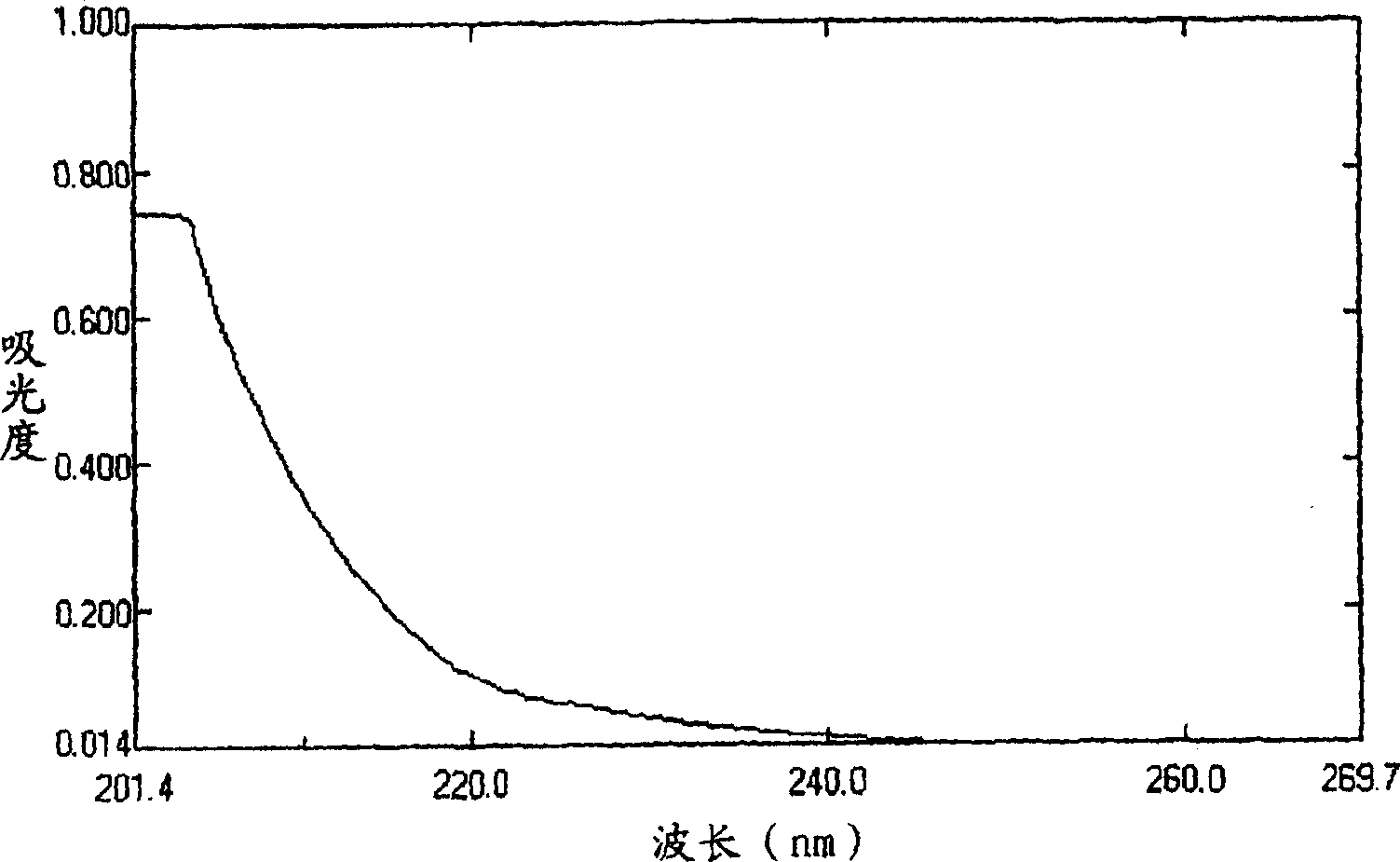
![1,2-Dioxane[3,4-f]Nitrooxycyclononane Derivatives and Their Synthesis and Application 1,2-Dioxane[3,4-f]Nitrooxycyclononane Derivatives and Their Synthesis and Application](https://images-eureka.patsnap.com/patent_img/3b9da713-a626-4eb3-befa-c0adb4cd9fd4/BDA0002312540640000011.png)
![1,2-Dioxane[3,4-f]Nitrooxycyclononane Derivatives and Their Synthesis and Application 1,2-Dioxane[3,4-f]Nitrooxycyclononane Derivatives and Their Synthesis and Application](https://images-eureka.patsnap.com/patent_img/3b9da713-a626-4eb3-befa-c0adb4cd9fd4/BDA0002312540640000021.png)
![1,2-Dioxane[3,4-f]Nitrooxycyclononane Derivatives and Their Synthesis and Application 1,2-Dioxane[3,4-f]Nitrooxycyclononane Derivatives and Their Synthesis and Application](https://images-eureka.patsnap.com/patent_img/3b9da713-a626-4eb3-befa-c0adb4cd9fd4/BDA0002312540640000041.png)

![A method for synthesizing 5-fluoro-1-azabicyclo[3,2,2]nonane and derivatives thereof A method for synthesizing 5-fluoro-1-azabicyclo[3,2,2]nonane and derivatives thereof](https://images-eureka.patsnap.com/patent_img/9da96b4a-b646-4fdf-b767-1470745b7e8d/119595DEST_PATH_IMAGE009.png)
![A method for synthesizing 5-fluoro-1-azabicyclo[3,2,2]nonane and derivatives thereof A method for synthesizing 5-fluoro-1-azabicyclo[3,2,2]nonane and derivatives thereof](https://images-eureka.patsnap.com/patent_img/9da96b4a-b646-4fdf-b767-1470745b7e8d/141461DEST_PATH_IMAGE005.png)
![A method for synthesizing 5-fluoro-1-azabicyclo[3,2,2]nonane and derivatives thereof A method for synthesizing 5-fluoro-1-azabicyclo[3,2,2]nonane and derivatives thereof](https://images-eureka.patsnap.com/patent_img/9da96b4a-b646-4fdf-b767-1470745b7e8d/163595DEST_PATH_IMAGE002.png)
![Method for synthesizing 5-fluoro-1-azabicyclo[3, 2, 2]nonane and derivatives thereof Method for synthesizing 5-fluoro-1-azabicyclo[3, 2, 2]nonane and derivatives thereof](https://images-eureka.patsnap.com/patent_img/a20ecb9c-28cb-46a4-ac42-46d9a8e19428/119595DEST_PATH_IMAGE009.png)
![Method for synthesizing 5-fluoro-1-azabicyclo[3, 2, 2]nonane and derivatives thereof Method for synthesizing 5-fluoro-1-azabicyclo[3, 2, 2]nonane and derivatives thereof](https://images-eureka.patsnap.com/patent_img/a20ecb9c-28cb-46a4-ac42-46d9a8e19428/141461DEST_PATH_IMAGE005.png)
![Method for synthesizing 5-fluoro-1-azabicyclo[3, 2, 2]nonane and derivatives thereof Method for synthesizing 5-fluoro-1-azabicyclo[3, 2, 2]nonane and derivatives thereof](https://images-eureka.patsnap.com/patent_img/a20ecb9c-28cb-46a4-ac42-46d9a8e19428/174008DEST_PATH_IMAGE001.png)