A kind of preparation method of macrolide precursor
A macrolide and precursor technology, which is applied in the field of preparation of macrolide precursors, can solve the problems of difficult control of reaction conditions and high degree of risk, and achieve convenient operation, easy-to-obtain substrate structure, and simple substrate structure Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
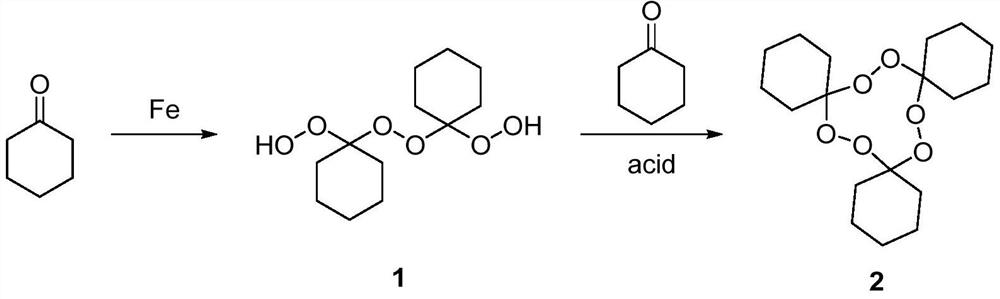
[0024] The preparation method of a kind of macrolide precursor 1,2,4,5,7,8-hexaoxa-3,6,9-tricyclohexylidene cyclononane of the present invention comprises the following steps:
[0025] (1) Mix cyclohexanone and hydrogen peroxide, add an iron salt catalyst, react at 20-45°C for 1-6h, extract and concentrate after the reaction, and separate to obtain the main product;
[0026] (2) At 20-45°C, react the main product of the previous step with cyclohexanone through an organic acid catalyst for 1-3 hours, and separate and purify after the reaction to obtain 1,2,4,5,7,8-hexaoxa- 3,6,9-Tricyclohexylcyclononane.
[0027] Reaction formula of the present invention is as follows:
[0028]
Embodiment 1
[0030] Weigh 10.0mmol of cyclohexanone and 30mmol of hydrogen peroxide into the bottle, add 2mmol of ferric ammonium sulfate dodecahydrate, react at 25°C for 1h, after the reaction is completed, after extraction and concentration, ethyl acetate / petroleum ether = 1 / 5(V / V) is the eluent, and the main product 1,1'-dihydroperoxydicyclohexyl peroxide is obtained by separation by column chromatography.
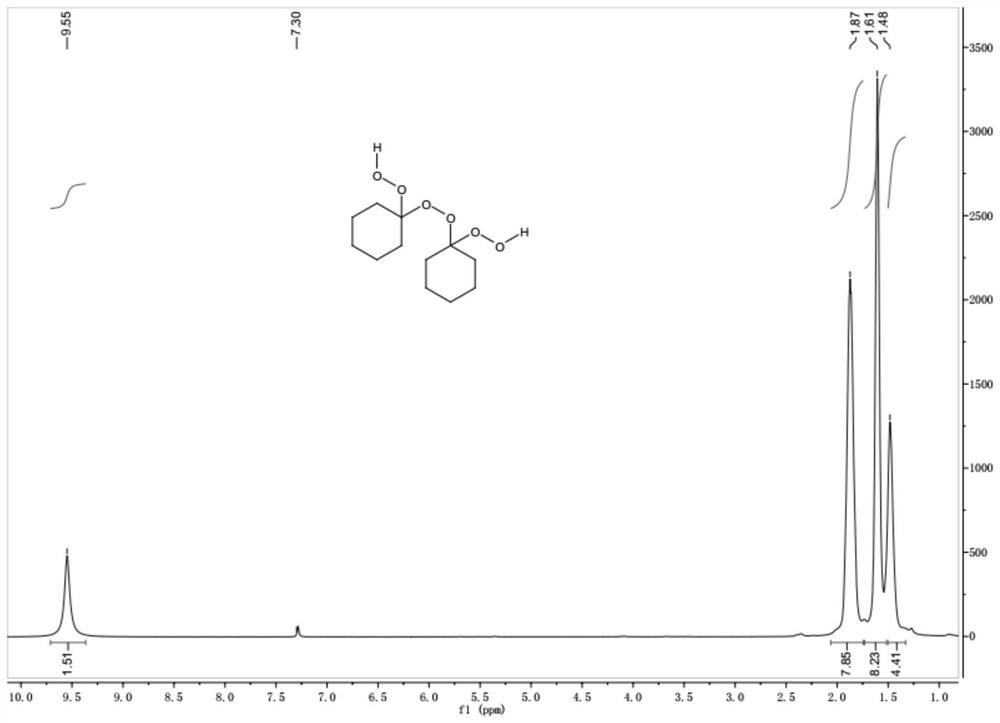
[0031] Such as figure 1 As shown, the main product 1 was characterized by NMR, and the results are as follows: 1 H NMR (500MHz, CDCl 3 )δ9.55 (s, 2H), 1.87 (s, 8H), 1.61 (s, 8H), 1.48 (s, 4H).
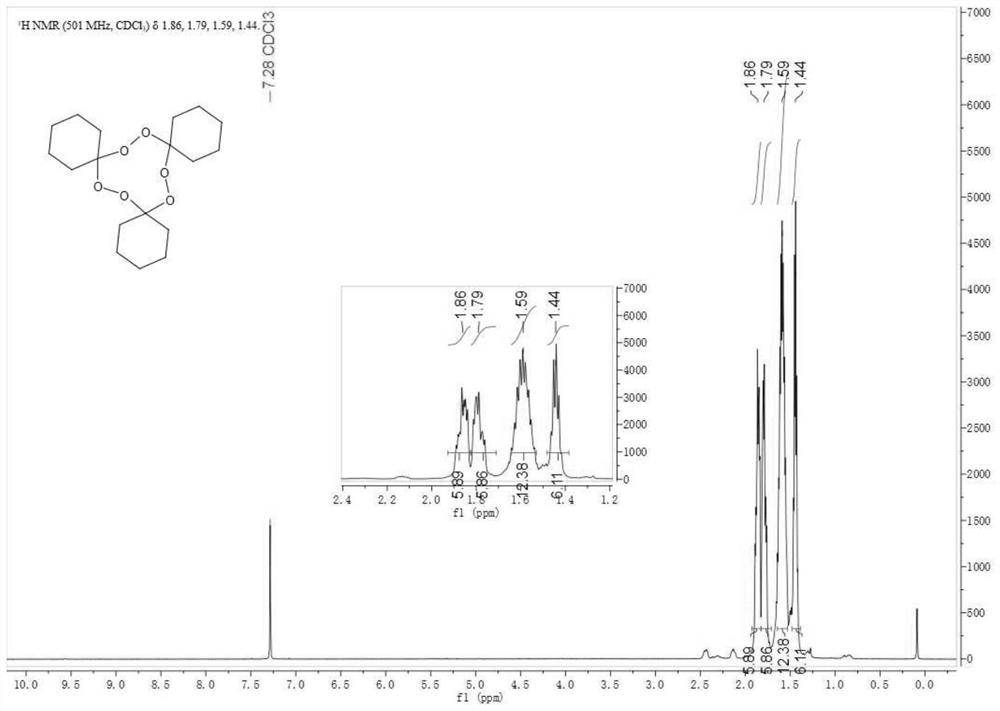
[0032] At 25°C, the main product was mixed with equimolar cyclohexanone, and p-toluenesulfonic acid was added (based on the total moles of the main product, cyclohexanone and p-toluenesulfonic acid, the molar percentage of p-toluenesulfonic acid was 5 %) was reacted for 2 h, ethyl acetate / petroleum ether=1 / 70 (V / V) was used as the eluent, and the product was separated by column chromatog...
Embodiment 2
[0036] Weigh 10.0mmol of cyclohexanone and 30mmol of hydrogen peroxide into the bottle, add 2mmol of iron sulfate, and react for 1h at 25°C. After the reaction, after extraction and concentration, ethyl acetate / petroleum ether=1 / 5(V / V) is the eluent, and the main product 1 is obtained by column chromatography separation. At 25° C., the main product is mixed with equimolar cyclohexanone, and p-toluenesulfonic acid (based on the main product, cyclohexanone and p-toluenesulfonic acid) is added. Add the total moles of benzenesulfonic acid as the basis, and the molar percentage of p-toluenesulfonic acid is 5%) to react for 2h, ethyl acetate / petroleum ether=1 / 70 (V / V) is the eluent, and is separated by column chromatography product.
[0037] The total yield of target product 2 in this embodiment 2 is 37%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com