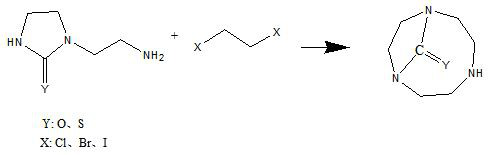
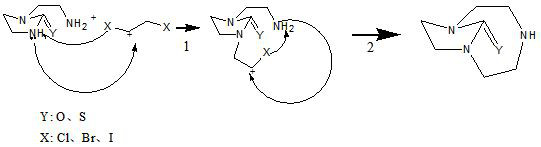
Synthetic method of 1,4,7-triazacyclononane-1,4-one and 1,4,7-triazacyclononane-1,4-thione
A technology of triazacyclononane and synthesis method, applied in the direction of organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0032] In a 1000ml four-necked flask equipped with a mechanical stirrer, a reflux condenser and a thermometer, 25.8g (0.2mol) of 1-(2-aminoethyl)-2-imidazolidinone, 1,2-dibromoethyl 37.5 g (0.2 mol) of alkane, 70 g (0.5 mol) of anhydrous potassium carbonate and 600 ml of acetonitrile. After the addition was complete, it was stirred at room temperature for 12 hours.
[0033] Then the oil bath was heated to reflux, the reflux temperature was 85° C., and the reflux was kept for 144 hours.
[0034] After keeping warm, cool to room temperature. Filtration, the filter cake is the reaction by-product potassium bromide and excess potassium carbonate. Rinse the filter cake with 100ml of fresh acetonitrile, and combine the filtrate and washings. The acetonitrile was evaporated under reduced pressure with a rotary evaporator, and the obtained orange-red oily liquid was heated to boiling by adding 100 ml of methanol, and then refrigerated overnight to precipitate white flaky crystals. ...
example 2
[0036] In a 1000ml four-necked flask equipped with a mechanical stirrer, a constant pressure dropping funnel, a reflux condenser and a thermometer, 600ml of N,N-dimethylformamide, 56g of N,N-diisopropylethylamine (0.43 mol), 1-(2-aminoethyl)-2-imidazolinone 25.8g (0.2mol), 1,2-dibromoethane 37.5g (0.2mol). After the addition was complete, it was stirred at room temperature for 12 hours.
[0037] Then the oil bath was heated to 100 degrees to keep warm for 48 hours.
[0038] After the heat preservation is over, transfer the reaction solution to a rotary evaporator and rotary evaporate under reduced pressure. After evaporating the solvent, add 500ml of dichloromethane and 100ml of water to the residue in the bottle and stir for 10 minutes. Separate the water layer and wash the oil layer with 100ml of water twice . The oil layer washed with water was dried over anhydrous sodium sulfate, and dichloromethane was distilled off to obtain a light yellow solid. It was recrystallized...
example 3
[0041] In a 1000ml four-necked flask equipped with a mechanical stirrer, a reflux condenser and a thermometer, 29g (0.2mol) of 1-(2-aminoethyl)-2-imidazolinthione, 1,2-dichloroethyl 19.8g (0.2mol) of alkane, 50.5g (0.5mol) of triethylamine, 2.5g of potassium iodide and 600ml of acetonitrile. After the addition was complete, it was stirred at room temperature for 72 hours.
[0042] Then the oil bath was heated to reflux, the reflux temperature was 84° C., and the reflux was kept for 96 hours.
[0043] After the heat preservation is over, transfer the reaction solution to a rotary evaporator and rotary evaporate under reduced pressure. After evaporating the solvent, add 500ml of dichloromethane and 100ml of water to the residue in the bottle and stir for 10 minutes. Separate the water layer and wash the oil layer with 100ml of water twice . The oil layer washed with water was dried over anhydrous sodium sulfate, and dichloromethane was distilled off to obtain a light yellow so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com