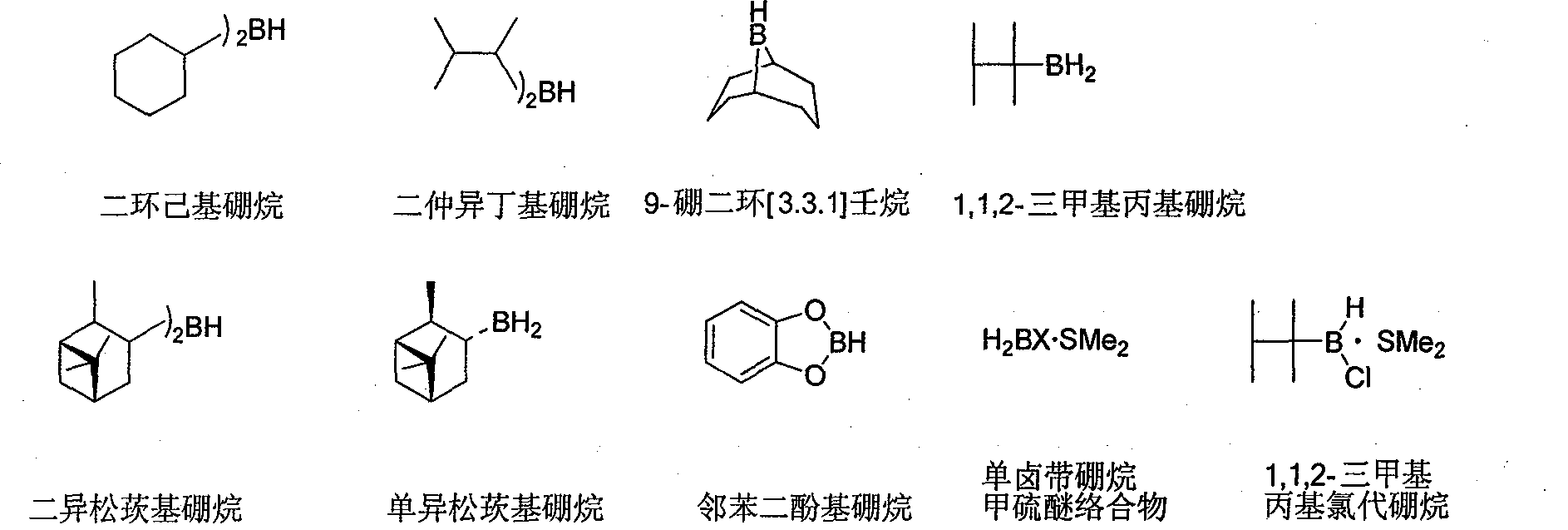
Preparation method of 9-boron bicyclo (3,3,1)-nonane (9-BBN)
A technology of 9-BBN and nonane, which is applied in the field of preparation of 9-boronbicyclo-nonane, can solve the problems that the preparation method is not easy to realize industrialized large-scale production, and achieve the advantages of shortening the product synthesis cycle, good application prospects, and facilitating industrialization Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0031] Under the protection of nitrogen, add 10g of crushed 4A molecular sieve, 100ml of solvent tetrahydrofuran, 12.3ml of 1,5-cyclooctadiene and 0.23g of zirconium tetrachloride in a 250ml reaction flask equipped with a reflux device, and magnetically stir at room temperature for 1 hour to make The reactants are evenly mixed, and the reaction solvent and trace water in the raw materials are removed. Cool down to about 0℃, add 10ml of 10M / L borane dimethyl sulfide complex dropwise at a rate of 1 drop per second. After the dropwise addition is complete, heat and reflux for 4 hours, take samples, and detect the isomer 9-boron by GC Bicyclo(4,2,1)-nonane is completely converted into 9-boronbicyclo(3,3,1)-nonane, stop heating, cool the obtained product to 0℃, continue stirring, keep it for 3 hours, It was filtered under the protection of nitrogen to obtain 11.9 g of crystalline solid with a yield of 98.0%.
Embodiment example 2
[0033] Under the protection of nitrogen, add 10g of crushed 4A molecular sieve, 100ml of solvent tetrahydrofuran, 12.3ml of 1,5-cyclooctadiene and 0.23g of zirconium tetrachloride in a 500ml reaction flask equipped with a reflux device, and magnetically stir at room temperature for 1 hour to make The reactants are evenly mixed, and the reaction solvent and trace water in the raw materials are removed. Cool down to about 0°C, add 100ml of 1M / L borane dimethyl sulfide complex at a rate of 5 drops per second. After the addition is complete, heat and reflux for 4 hours, take samples, and detect the isomer 9-boron by GC Bicyclo(4,2,1)-nonane is completely converted into 9-boronbicyclo(3,3,1)-nonane, stop heating, cool the obtained product to 0℃, continue stirring, keep it for 3 hours, It was filtered under the protection of nitrogen to obtain 11.6 g of crystalline solid with a yield of 95.2%.
Embodiment example 3
[0035] Under the protection of nitrogen, add 10g of crushed 4A molecular sieve, 100ml of 1,4-dioxane solvent, 12.3ml of 1,5-cyclooctadiene and 1.1g of zirconium tetrachloride into a 500ml reaction flask equipped with a reflux device in sequence, at room temperature Magnetic stirring is applied for 1 hour to make the reactants mix uniformly and remove trace water in the reaction solvent and raw materials. Cool down to about 0℃, add 10ml of 10M / L borane dimethyl sulfide complex dropwise at a rate of 1 drop per second. After the dropwise addition is complete, heat and reflux for 4 hours, take samples, and detect the isomer 9-boron by GC Bicyclo(4,2,1)-nonane is completely converted into 9-boronbicyclo(3,3,1)-nonane, stop heating, cool the obtained product to 0℃, continue stirring, keep it for 3 hours, It was filtered under the protection of nitrogen to obtain 11.8 g of crystalline solid with a yield of 96.7%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com