"One-pot" synthesis of irbesartan intermediate
A technology for intermediates and compounds, applied in the field of antihypertensive drugs, can solve problems such as unfavorable industrial production, and achieve the effects of convenient operation, high yield and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
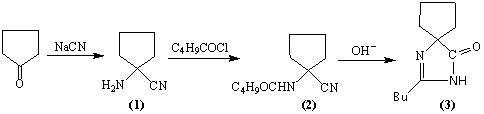
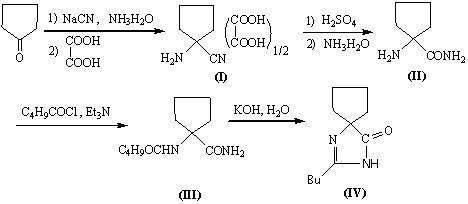
[0019] Example 1: 20g sodium cyanide (0.41mol) is dissolved in 40mL water, add 60mL aqueous solution containing 23g ammonium chloride (0.43mol), 35mL ammonia water and 40mL methanol solution containing 30g cyclopentanone (0.36mol), stir at room temperature After 1.5h, the temperature was raised to 60°C and stirred for another 45min, then the heating was stopped, and the stirring was continued for 45min. Cool to 25°C, extract with dichloromethane (80ml×6), combine organic phases, dry over anhydrous magnesium sulfate, and filter to obtain a dichloromethane solution containing 39g of 1-aminocyclopentanylcyanide (1). Add 39g (0.385mol) of triethylamine to the above solution, cool to 20°C, add 42g (0.348mol) of n-valeryl chloride dropwise, the temperature rises to 35°C and stir for 2 hours. , 80mL of 2% hydrochloric acid water and 100mL of water, distilled off dichloromethane to obtain about 63g of brown oil compound (2). Compound (2) was added to a solution of 200mL methanol and...
Embodiment 2
[0020] Embodiment 2: 20g sodium cyanide (0.41mol) is dissolved in 40mL water, add the 60mL aqueous solution that contains 23g ammonium chloride (0.43mol), 35mL ammoniacal liquor and the 40mL methanol solution that contains 30g cyclopentanone (0.36mol), stir at room temperature After 1.5h, the temperature was raised to 60°C and stirred for another 45min, then the heating was stopped, and the stirring was continued for 45min. Cool to 25°C, extract with toluene (80ml×6), combine organic phases, dry over anhydrous magnesium sulfate, and filter to obtain a toluene solution containing 39g of 1-aminocyclopentacyanide (1). Add 39g (0.385mol) of triethylamine to the above solution, cool to 20°C, add 42g (0.348mol) of n-valeryl chloride dropwise, the temperature rises to 50°C and stir for 2 hours. , 80mL of 2% hydrochloric acid water and 100mL of water, and vacuum evaporated toluene to obtain about 64g of brown oil compound (2). Add compound (2) to a solution of 200mL methanol and 80m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com