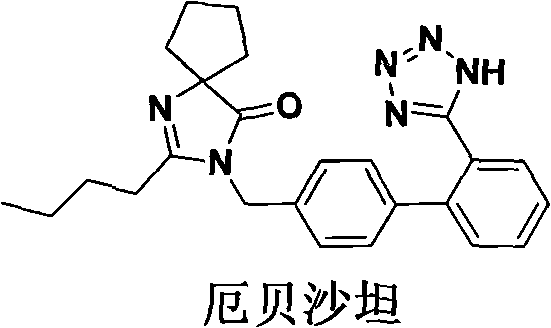
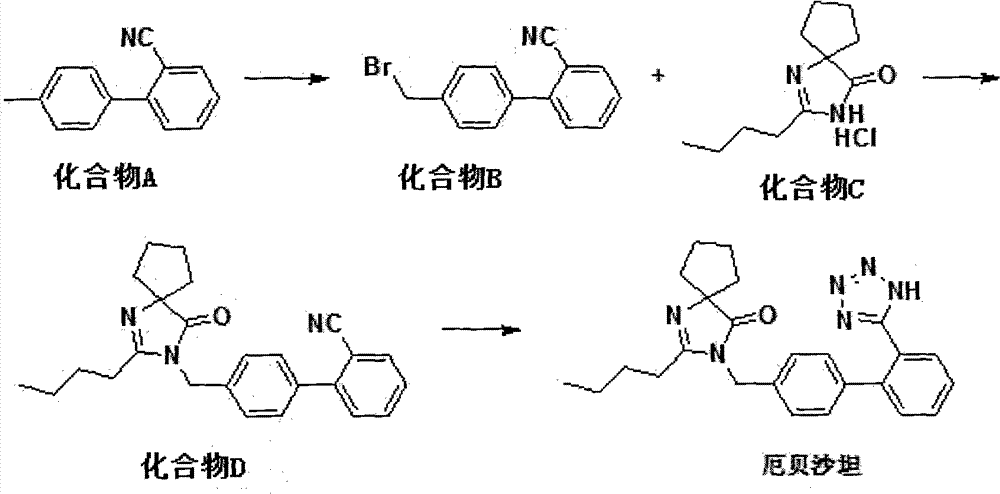
Synthetic route and preparation method of irbesartan
A compound and reaction technology, applied in the field of drug preparation, can solve the problems of long and tedious routes, safety risks and high costs, and achieve the effects of shortened reaction time, short reaction routes and high purity products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Add compound A (116g, 0.6mol) in a 5L three-necked flask, add 1200mL DCM, stir to dissolve, then weigh NaBrO 3 (180g, 1.20mol) dissolved in 600mLH 2 O, added to the above reaction system, stirred, and then weighed Na 2 S 2 o 4 (209g, 1.20mol) was dissolved in 1200mL of water, transferred to a constant pressure dropping funnel, and slowly added dropwise to the above solution. After the addition, the reaction was allowed to react at room temperature for 4h, and monitored by TLC until the reaction was complete. Sodium bisulfite solution was added dropwise at 25°C until the solution was pale yellow or colorless, and the layers were separated after standing. The aqueous phase was extracted with 400 mL of dichloromethane, and the organic phase was combined, washed twice with 800 mL of purified water, and the organic phase was vortexed. After drying, add ethyl acetate and n-hexane mixture, stir and crystallize, filter, and drain to obtain a white solid product, which is vac...
Embodiment 2
[0044] Add compound A (116g, 0.6mol) in a 5L three-necked flask, add 1200mL chlorobenzene, stir to dissolve, then weigh NaBrO 3 (180g, 1.20mol) was dissolved in 600mLH2O, added to the above reaction system, stirred, and then weighed Na 2 S 2 o 4 (209g, 1.20mol) was dissolved in 1200mL of water, transferred to a constant pressure dropping funnel, and slowly added dropwise to the above solution. After the addition, the reaction was allowed to react at room temperature for 4h, and monitored by TLC until the reaction was complete. Add sodium bisulfite solution dropwise at 250C until the solution is light yellow or colorless, let stand to separate layers, extract the aqueous phase with 400 mL of ethyl acetate, combine the organic phase, wash twice with 800 mL of purified water, and spin dry the organic phase , adding ethyl acetate and n-hexane mixture, stirring and crystallizing, filtering, and drying to obtain a white solid product, which was dried in vacuo at 400C for 2 hours, ...
Embodiment 3
[0046] Add compound C (46g, 0.2mol), compound B (54g, 0.2mol), dichloromethane 400mL, tetrabutylammonium bromide (0.7g, 0.002mol) into a 1L three-necked flask, stir and add sodium hydroxide (40g , 1mol) of 200mL aqueous solution, reacted at room temperature for 3h after the addition, and monitored by TLC until the reaction was completed. Stand to separate the layers, extract the aqueous phase with dichloromethane (100mL), combine the dichloromethane phases, wash twice with purified water, dry, spin dry the organic phase under reduced pressure, add ethyl acetate to dissolve, and then analyze with n-hexane. crystallized, filtered, collected the white solid product, and dried in vacuo at 40° C. for 2 h to obtain the product compound D (69.4 g), with a yield of 90% and a purity of 99% by HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com