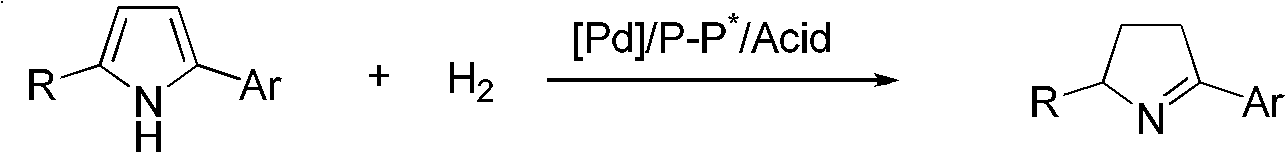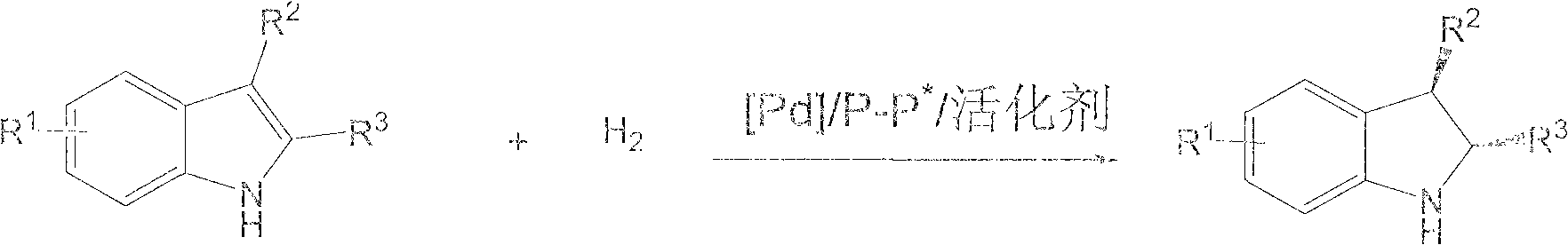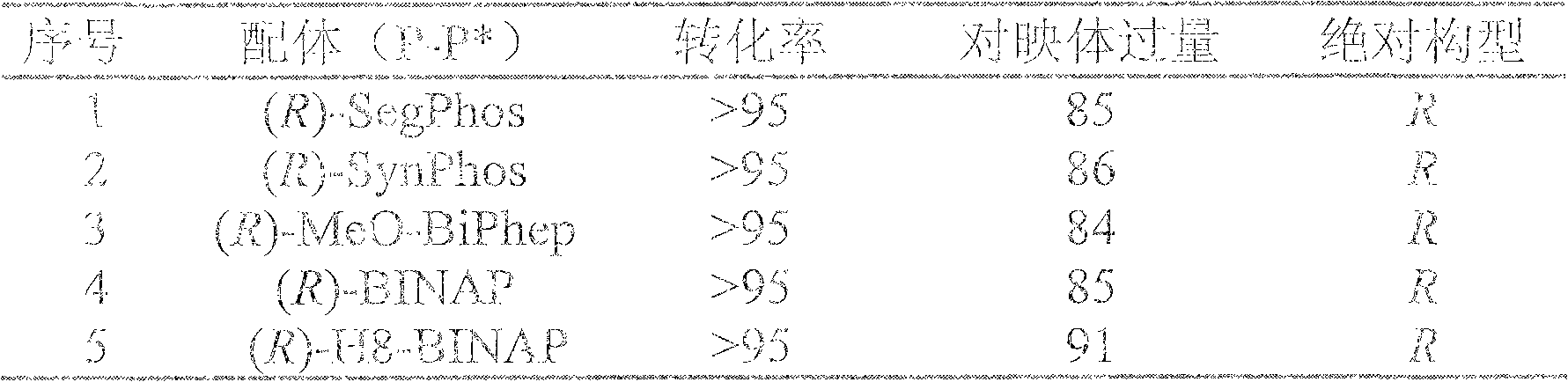Patents
Literature
30results about How to "High purity product" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
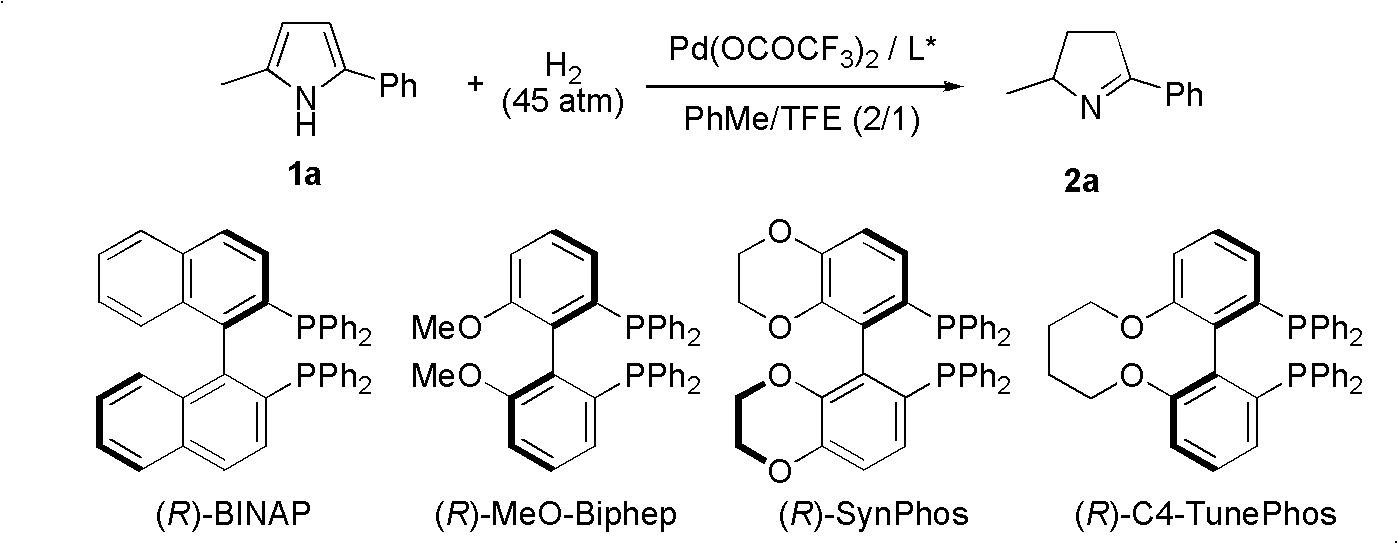
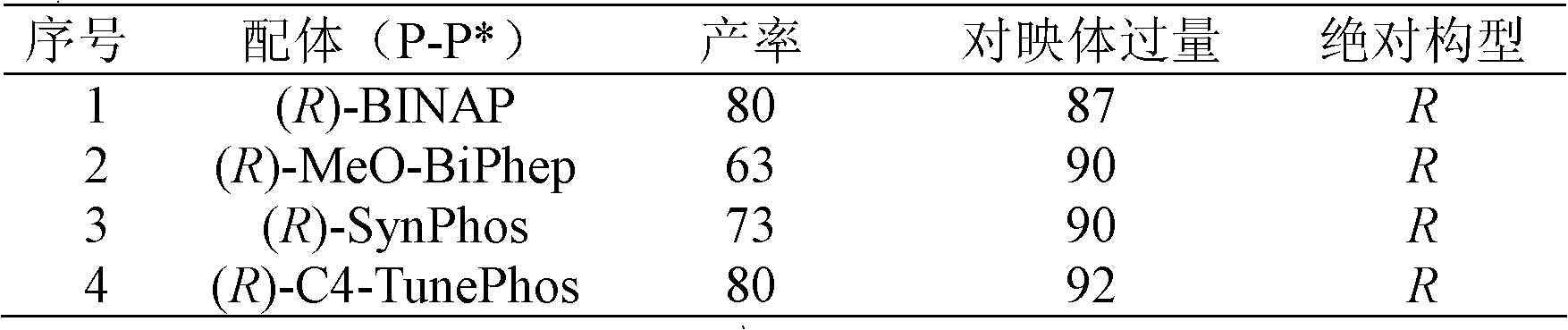
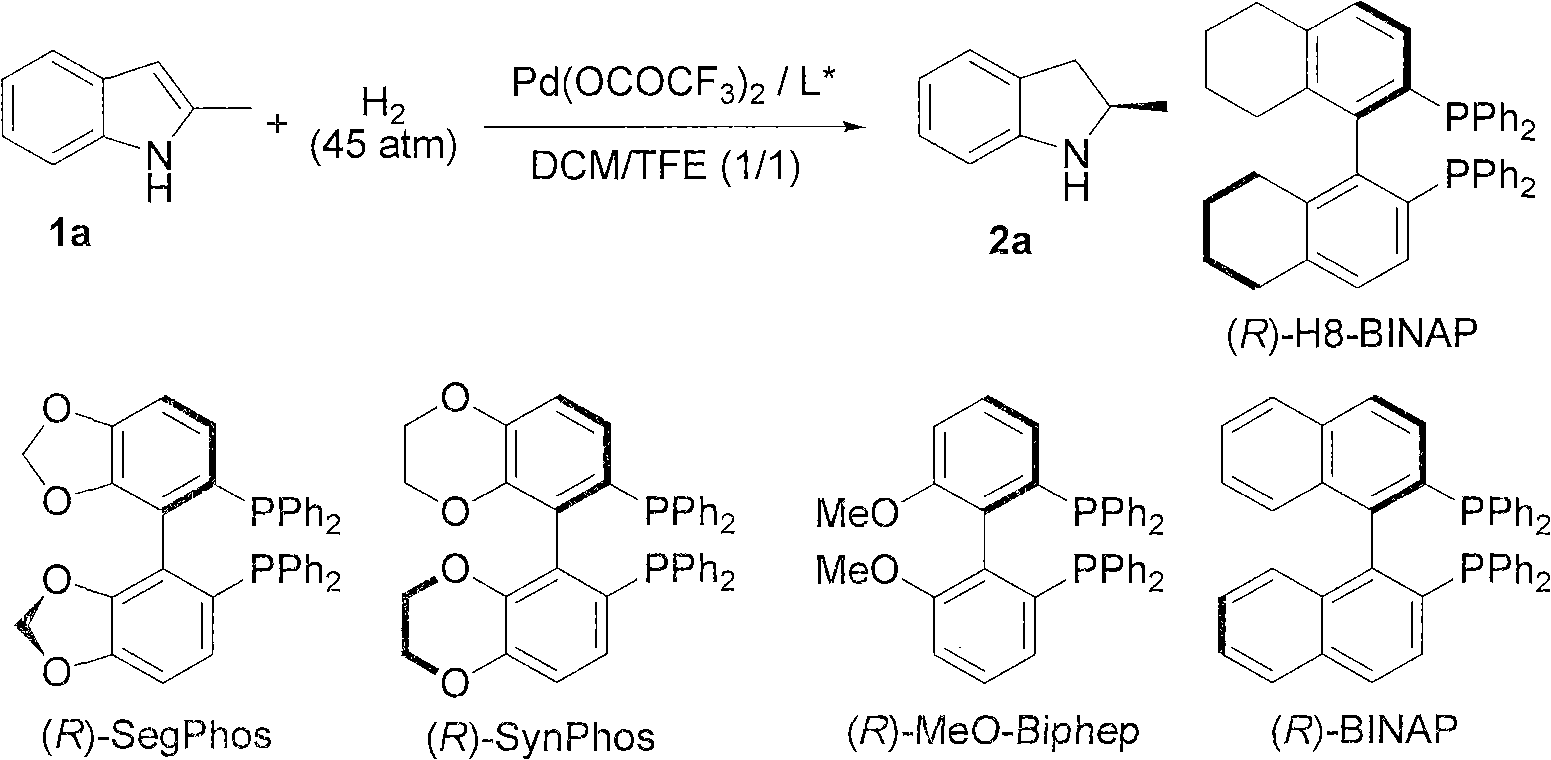
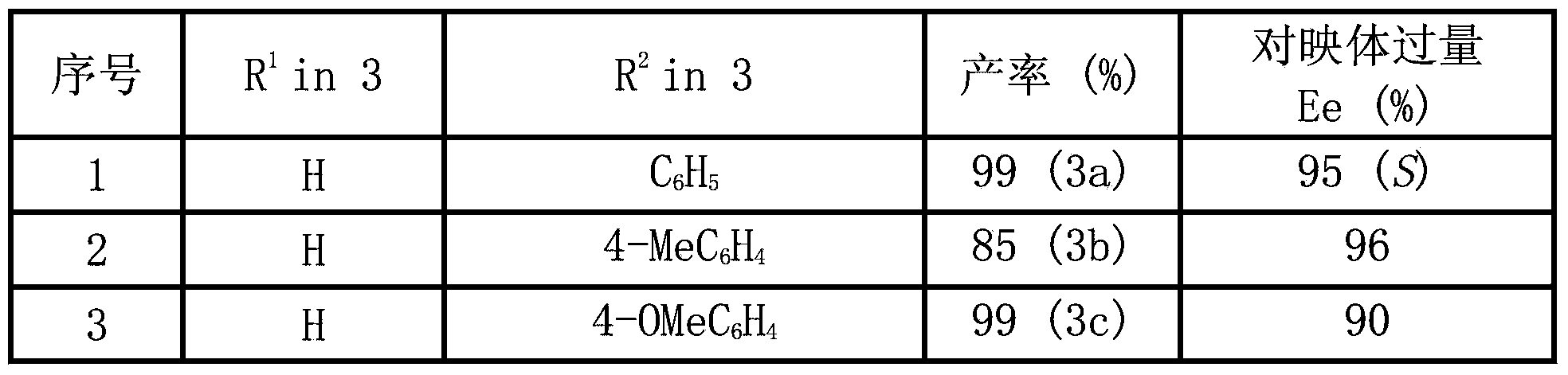
Chiral pyrroline synthetic method by palladium-catalyzed asymmetric hydrogenation
InactiveCN102838522AAsymmetric hydrogenationHigh enantioselectivityOrganic chemistryHydrogen pressureEnantiomer
A chiral pyrroline synthetic method by palladium-catalyzed asymmetric hydrogenation is characterized by using a Bronsted acid as an activator, and a catalyst system of a chiral diphosphine complex of palladium. The reaction can be carried out under the following conditions: a reaction temperature at 20-70 DEG C; a reaction solvent of a mixed solvent containing toluene and 2, 2, 2-trifluoroethanol (a volume of PhMe to TFE is 2:1); hydrogen pressure at 13-45 atmospheric pressure; a ratio of a substrate to the catalyst being 50 / 1; a metal precursor of Pd (OCOCF3)2; a chiral ligand of a chiral diphosphine ligand; and the activator of ethyl sulfonic acid (EtSO3H). Unprotected simple 2, 5 bisubstituted pyrrole is subjected to hydrogenation to obtain a corresponding chiral pyrroline product (1-pyrroline), and the enantiomer of the chiral pyrroline product can reach an excessive amount of 92%. The invention has advantages of simple operation, practicality, easily available raw materials, good enantioselectivity and high yield; besides, the reaction product is convenient for subsequent conversion.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for synthesizing chiral indoline through palladium-catalyzed asymmetric hydrogenation
InactiveCN102336698AAsymmetric hydrogenationHigh reactivityOrganic-compounds/hydrides/coordination-complexes catalystsAsymmetric synthesesHydrogen pressureReaction temperature
The invention relates to a method for synthesizing chiral indoline through palladium-catalyzed asymmetric hydrogenation, which is characterized in that Bronsted acid is used as the activator and the used catalysis system is a palladium chiral diphosphine complex. The reaction can be carried out under the following conditions: the reaction temperature is 20-50 DEG C; the solvent used in the reaction is a mixed solvent of dichloromethane and 2,2,2-trifluoroethanol (the DCM / TFE volume ratio is 1:1); the hydrogen pressure is 13-45 atmospheres; the substrate-catalyst ratio is 50 / 1; the used metal precursor is palladium trifluoroacetate (Pd(OCOCF3)2); the used chiral ligand is a chiral diphosphine ligand; the used activator is L-camphorsulfonic acid (L-CSA); and unprotected simple 2-substituted indole and 2,3-disubstituted indole can be hydrogenated to obtain the corresponding chiral indoline product, and the enantiomeric excess can be up to 96%. The method has the advantages of convenient operation process, high practicality, easy acquisition of raw materials, good enantioselectivity and high yield.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Apparatus and process for vacuum sublimation
InactiveUS20050047979A1High purity productAppropriate product purityVapor condensationSublimationEvaporationCompound (substance)
A vertical and a horizontal vacuum sublimation apparatus with high efficiency and processes thereof are provided, especially for the materials having high melting point and low vapor pressure. The vertical sublimation purification apparatus comprises a sublimation channel body, a material rack, a heating evaporation device, a condensation device, an incubating device and a product scratching device. The horizontal sublimation purification apparatus comprises a sublimation channel body, a material carrier, a heating evaporation device and two end pipes. The apparatuses of the present invention can be applied on high purity chemicals from mass production, and are capable of sublimating and purifying OLED illumination layer materials including ALq3, NPB and CuPc, which have high melting temperature and low vapor pressure.
Owner:IND TECH RES INST
Method for producing halogenated caesium lead material for perovskite solar cell
InactiveCN106449990ASimple processHigh purity productSolid-state devicesSemiconductor/solid-state device manufacturingChemical reactionHole transport layer
The invention relates to a method for producing a halogenated caesium lead material that is obtained based on solid-liquid two-phase chemical reaction of a halogenated ammonium lead material and cesium carbonate and has advantages of low production cost, high stability and easy dispersion. A halogenated ammonium lead, cesium carbonate, and C1-C4 fatty alcohol are mixed uniformly according to a molar ratio 1 to 0.5-0.55 to 10-20; while heating is carried out slowly at a temperature of 70 to 110 DEG C, reaction is carried out for 2 to 6 hours, so that the cesium carbonate reacts with the halogenated ammonium lead material to obtain a halogenated caesium lead material and ammonium carbonate as a by product decomposes and volatilizes; and a reaction product is cooled to be reach a temperature of 50 to 60 DEG C, a polar solvent as a cosolvent of a halogenated caesium lead crystal and silicone oil as a surface treating agent are added, a supernatant solution is stirred continuous for 6 to 12 hours, thereby forming a halogenated caesium lead crystal particle with characteristics of uniform particle diameter and surface hydrophobicity. The method provided by the invention has characteristics of simple process and secure environment protection and is easy to expanded produce and industrial produce. The product obtained by using the method can be used as a material for a light absorption layer of a perovskite solar cell and a material of a hole transport layer.
Owner:TIANJIN VOCATIONAL INST
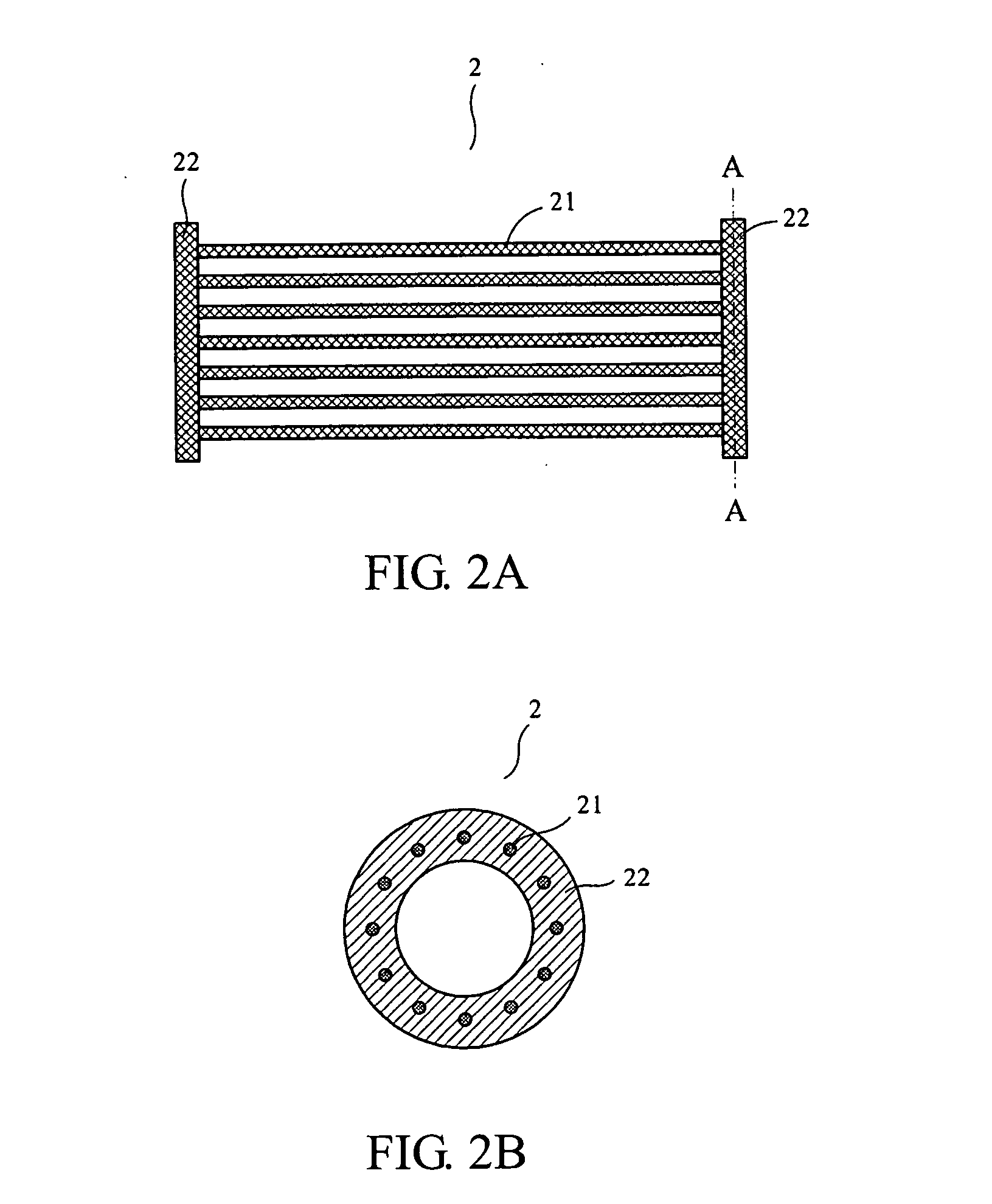
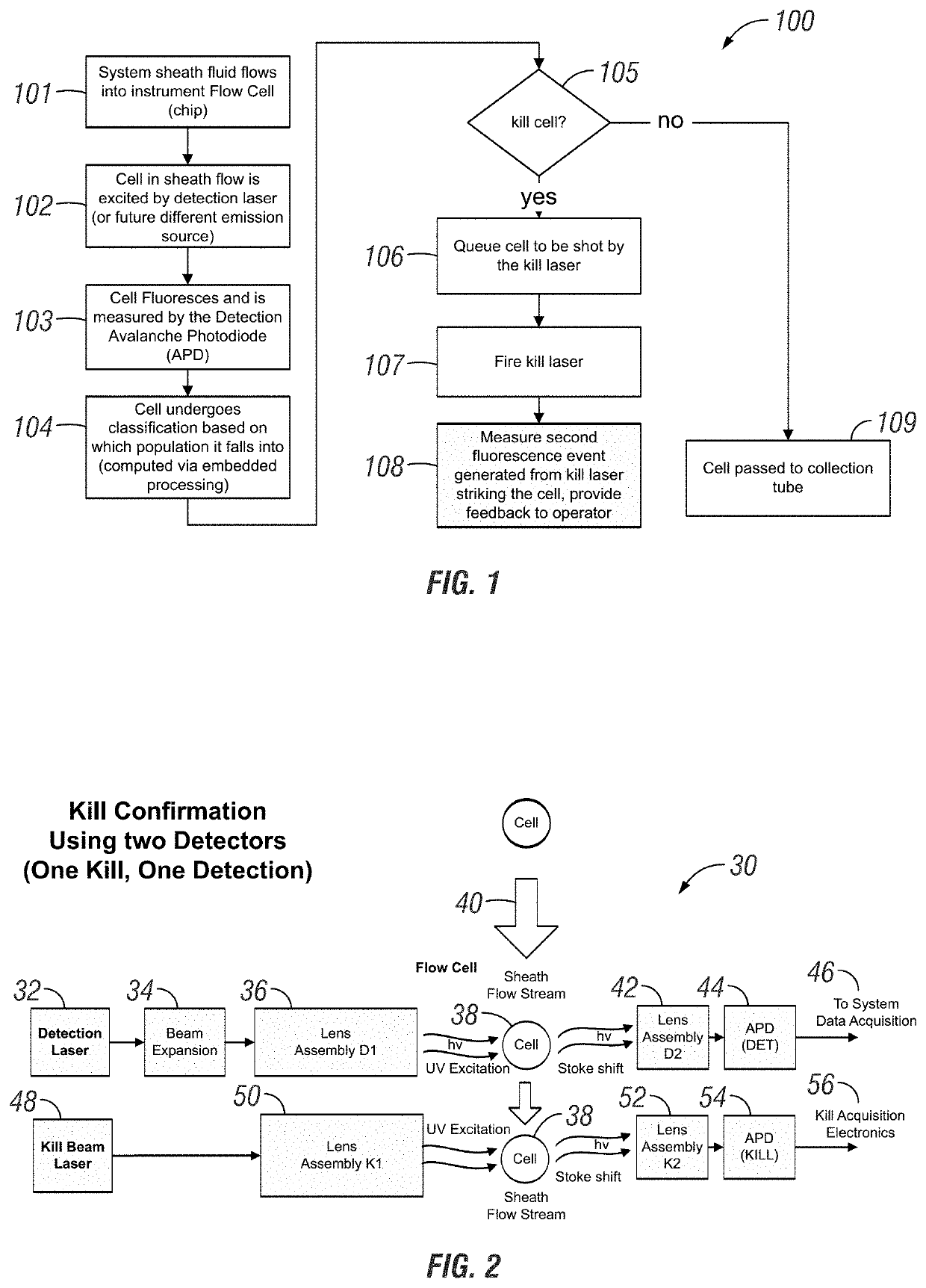
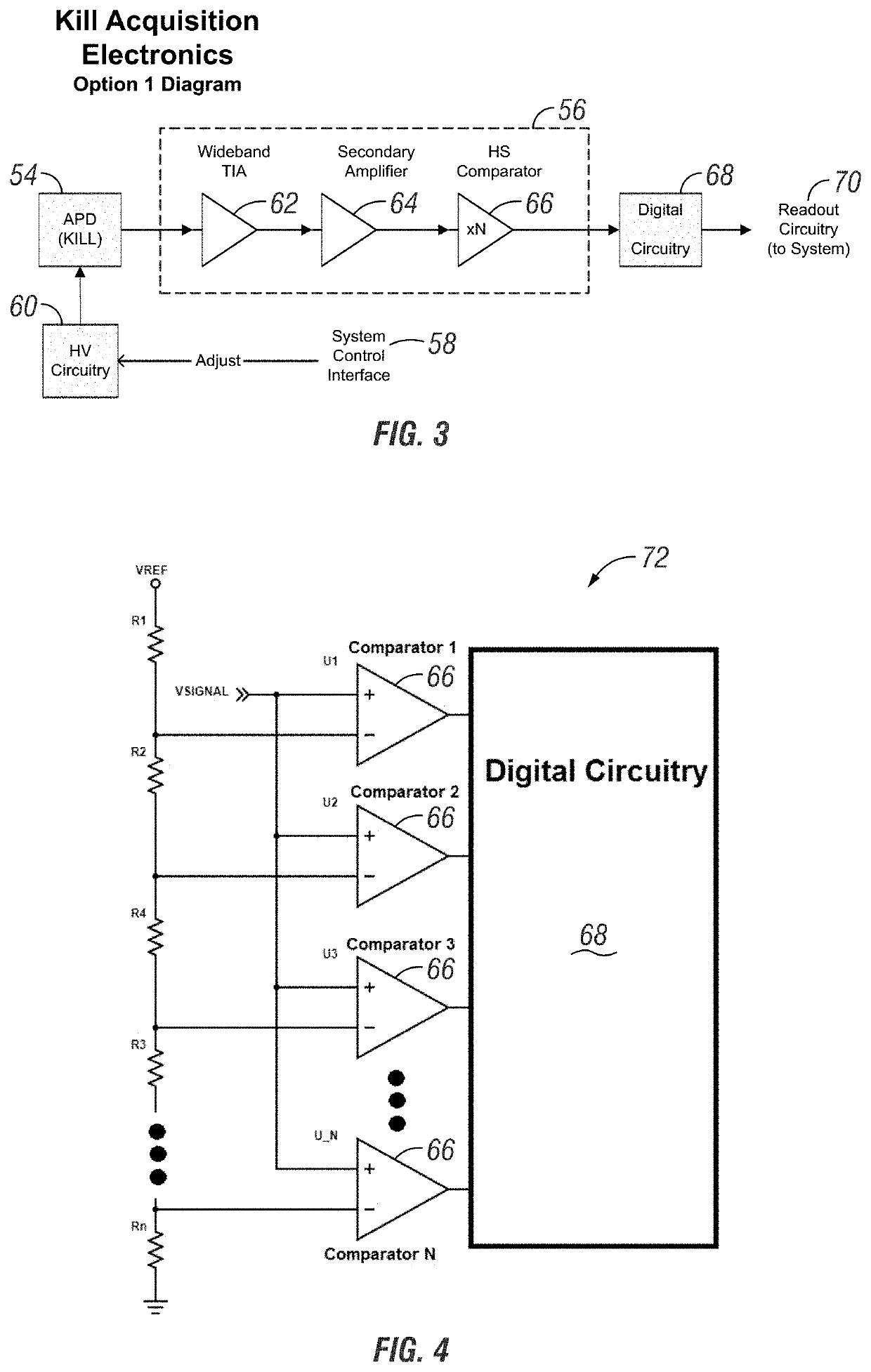
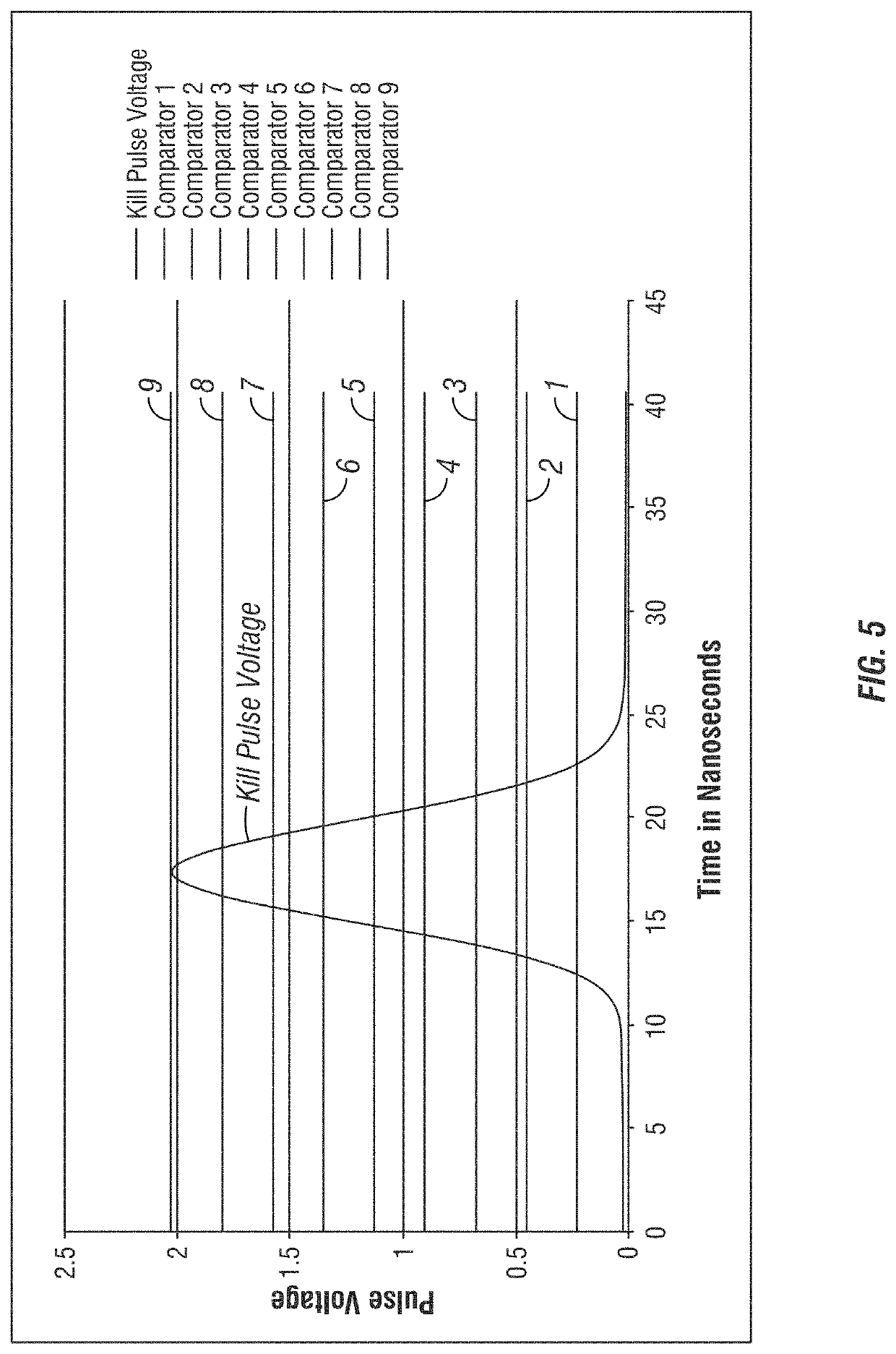
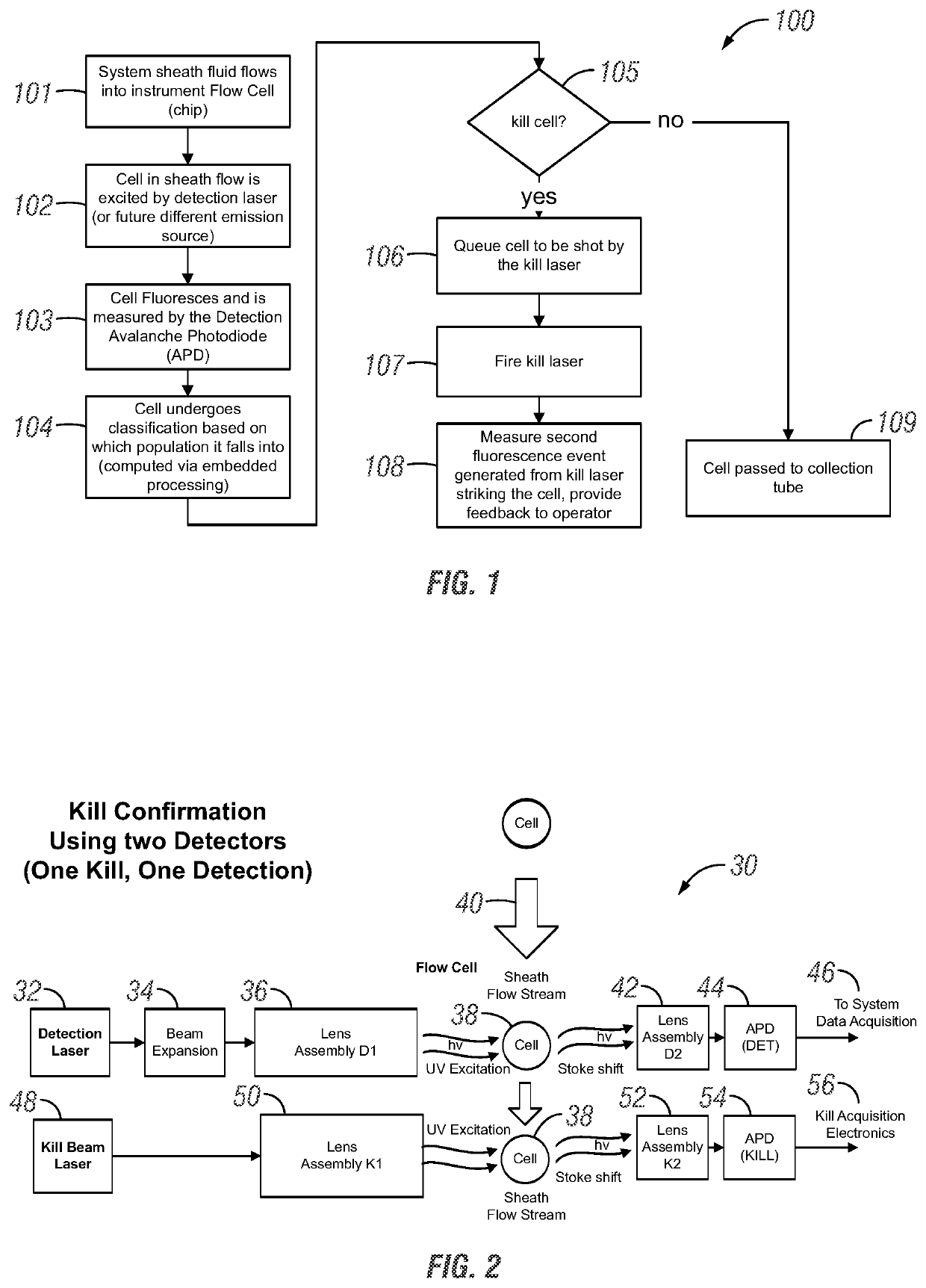
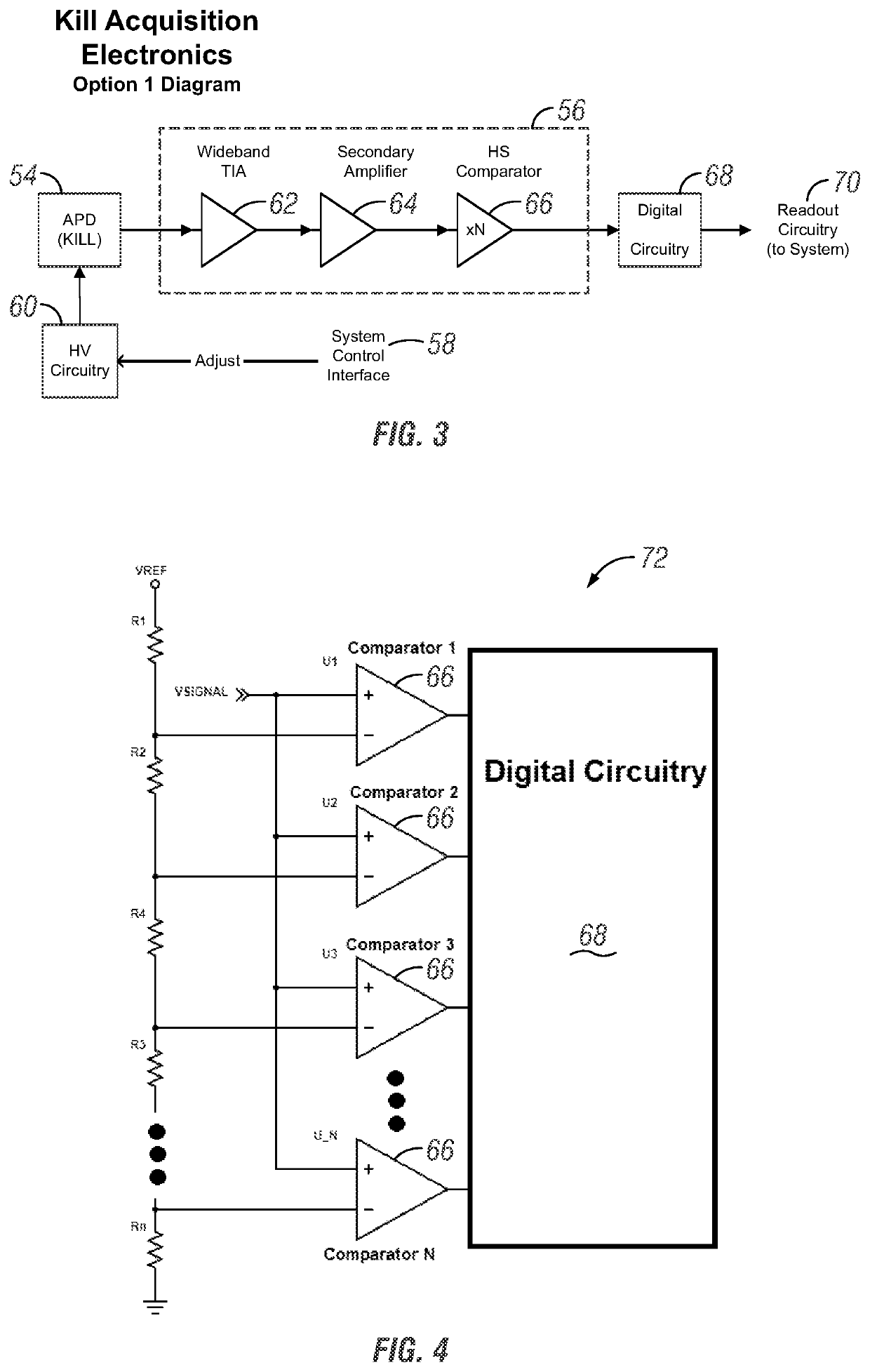
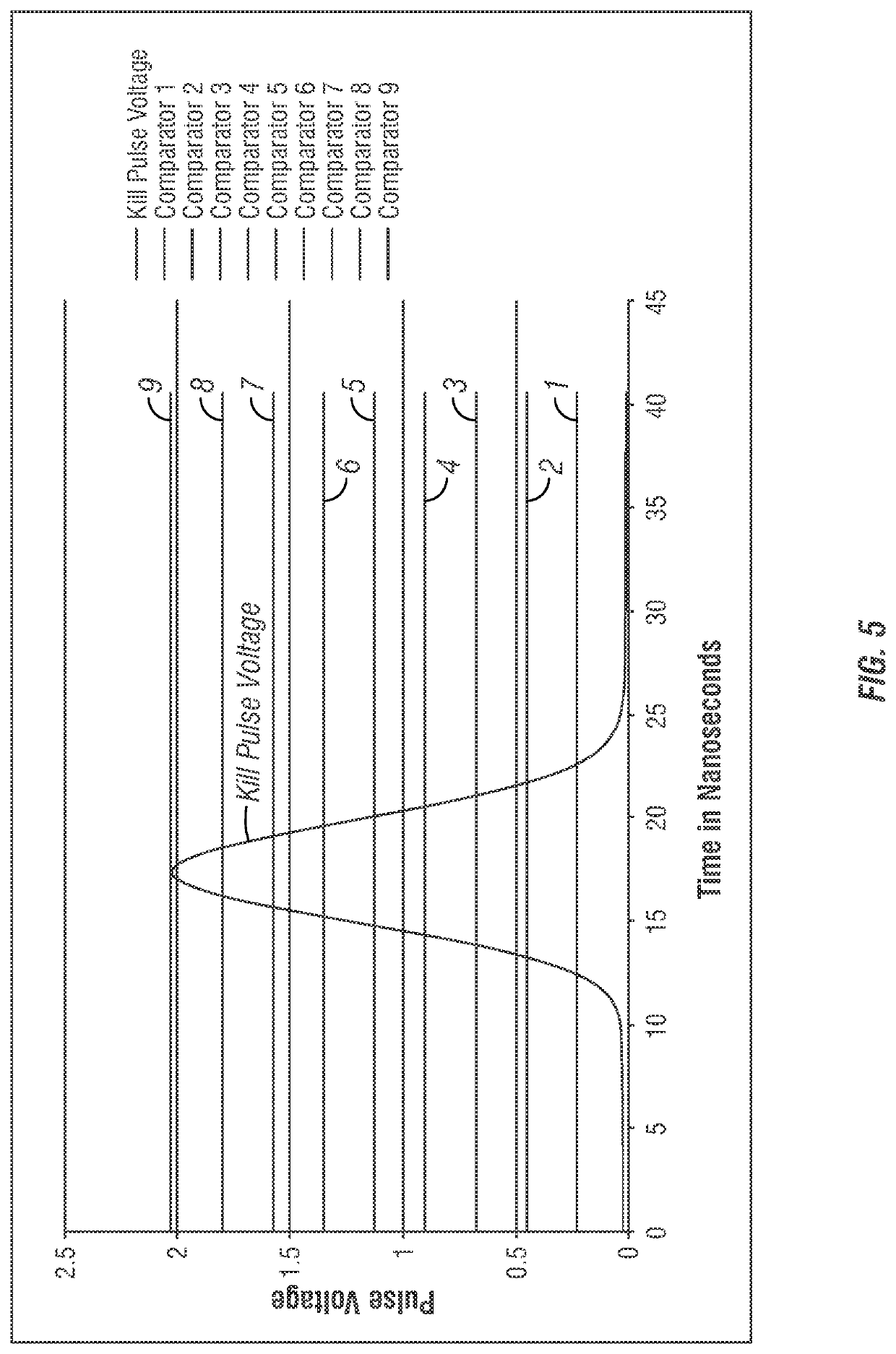
Apparatus and method for cell kill confirmation
ActiveUS20190382720A1Improve on and overcome deficiencyHigh purity productMicroscopesIndividual particle analysisFluorescenceCell based
A method and related apparatus for confirming whether a kill laser successfully destroys an undesired population of cells includes introducing fluorescent dye into cells, exciting the cells with a detection laser or a light emitting diode to cause the cell to fluoresce for a first time, measuring the amount of fluorescence in the cells with a detector capable of emitting a detection pulse, classifying the cells via embedded processing as undesired or desired cells based on the amount of fluorescence, firing a kill beam with a kill laser at any undesired cells, measuring the amount of fluorescence in the cells a second time to determine whether a fluorescent event was generated from the kill beam striking the cells, and providing feedback to an operator of the kill laser as to whether any fluorescent events were generated from the kill beam striking the cells.
Owner:ABS GLOBAL
Process for production of iodine compounds and process for production of high-purity 5-iodo-2-methylbenzoic acid
InactiveCN1747910AHigh purity productHigh purityCarboxylic acid nitrile preparationMolecular sieve catalystsOrganic solventChemical products
Provided is a production method for an iodine compound in which iodine is reacted with a substrate in the presence of a porous material having a pore diameter of 500 nm or less or in the presence of the above porous material and an oxidizing agent and a production process for high purity 5-iodo-2-methylbenzoic acid comprising an iodination reaction step carried out by the above-mentioned, a crystal precipitation and separation step in which a product is precipitated by adding water or cooling and then separated and a purification step in which crystal separated is recrystallized using an organic solvent. According to the production method for an iodine compound described above, iodine can be introduced into various substrates at a high selectivity. Since expensive metals and specific reagents do not have to be used, it can readily be carried out in an industrially scale, and the product having a high purity can be obtained. Further, the process comprising the iodination reaction, separation and purification steps described above makes it possible to readily obtain at a high yield, 5-iodo-2-methylbenzoic acid having a high purity which is useful in uses for functional chemical products such as medicines. The process of the present invention comprising iodination reaction, separation and purification steps is characterized by that it is simple in terms of a procedure and that the purification load is smaller, and it is very advantageous in industrially carrying out.
Owner:日本精细化工株式会社
Immobilized ionic-liquid catalyst and application thereof to synthesizing ester lubricating oil
ActiveCN105879907AThe synthesis method is simpleHigh catalyst activityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsTetraethyl orthosilicateEthyl ester
The invention discloses an immobilized ionic-liquid catalyst. A preparing method of the immobilized ionic-liquid catalyst includes the following steps that 1, ionic liquid, absolute ethyl alcohol and tetraethyl orthosilicate are stirred at the constant temperature of 40 DEG C to 70 DEG C till the ionic liquid is dissolved, and the solution is clarified; then a hydrochloric acid solution is added, the mixture is continuously stirred for 1 h to 3 h to be stood and aged for 12 h to 24 h, and a primary product of the mesoporous-silica immobilized ionic-liquid catalyst is obtained; 2, the primary product of the mesoporous-silica immobilized ionic-liquid catalyst is subjected to vacuum drying for 3 h to 6 h at the temperature of 80 DEG C to 100 DEG C, and the mesoporous-silica immobilized ionic-liquid catalyst is obtained. The invention further discloses an application of the catalyst to synthesizing ester lubricating oil. According to the immobilized ionic-liquid catalyst and the application thereof to synthesizing the ester lubricating oil, the catalyst is simple in the synthesizing method and high in catalyst activity, and the process of industrial applications of the catalyst is greatly quickened.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method of producing methylamine lead halide for perovskite solar cells
InactiveCN106410044AHigh purityHigh purity productFinal product manufactureSolid-state devicesFatty alcoholParaformaldehyde
The invention relates to a method of producing methylamine lead halide for perovskite solar cells at low cost through solid phase reaction of formaldehyde and ammonium lead halide. Halogen acid, ammonium halide, lead acetate and acetic acid are mixed according to the mole ratio of (2.1-2.1):(1.1-1.2):1:(20-40), reflux reaction is carried out after the mixture is heated to 100-120 DEG C, acetic acid and water are separated through vacuum distillation, and an ammonium halide lead crystal is generated. The ammonium halide lead crystal and paraformaldehyde are mixed according to the mole ratio of 1:(2.1-2.5), are slowly heated at 120-160 DEG C to react for 1-6h, and are further heated to 170-180 DEG C to react until there is no obvious gas emission. The reaction product is re-crystallized with polarity organic solvent, washed with fatty alcohol and vacuum-dried to get refined methylamine lead halide. The yield was 95-99.2%, and the content is 99.0-99.5%. The production technology is simple, environment-friendly and safe. There is no need for annealing treatment after coating. The methylamine lead halide can be directly used as a light absorption layer of a solar cell.
Owner:TIANJIN VOCATIONAL INST
Method for preparing lamellar-form borate crystal material
ActiveCN102963902ASave raw materialsHigh purity productPolycrystalline material growthFrom normal temperature solutionsEthylic acidVacuum drying
The invention discloses a method for preparing a lamellar-form borate crystal material, which comprises the following steps: dissolving borax and an ethylic acid in distilled water, then transferring the solution into a teflon-lined hydrothermal kettle; after constant-temperature heating reaction, naturally cooling the reaction product to room temperature; sequentially carrying out centrifugal washing on the obtained product in the hydrothermal kettle separately by using distilled water and absolute ethyl alcohol; and after centrifugal separation, carrying out vacuum drying so as to obtain a high-purity lamellar-form borate crystal material. Compared with borate crystals prepared by using traditional methods, borate crystals prepared by using the method disclosed by the invention has the advantages that raw materials are cheap, purity is high, grain size distribution is narrow, grain agglomeration degree is low, crystal growth is complete, form and size are controllable, the process is simple and efficient, preparation temperature is low, later crystallization does not need to be carried out, and the defects of grain growth, coarsening or curling and the like possibly caused in the process of later heat treatment can be avoided to a certain extent.
Owner:吴文婕
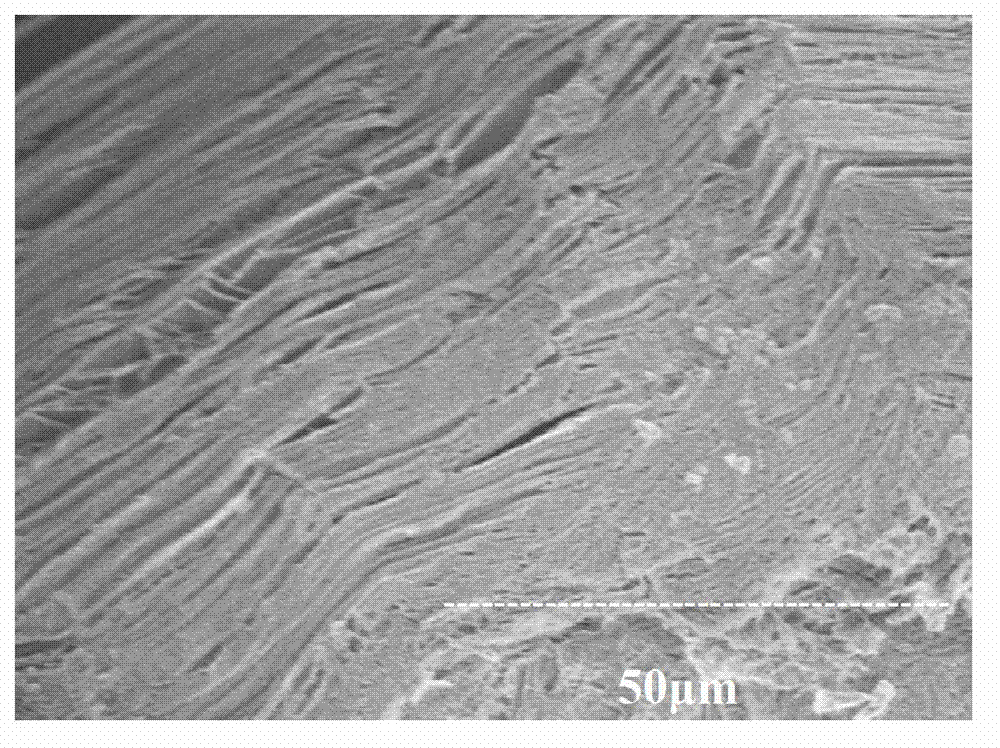
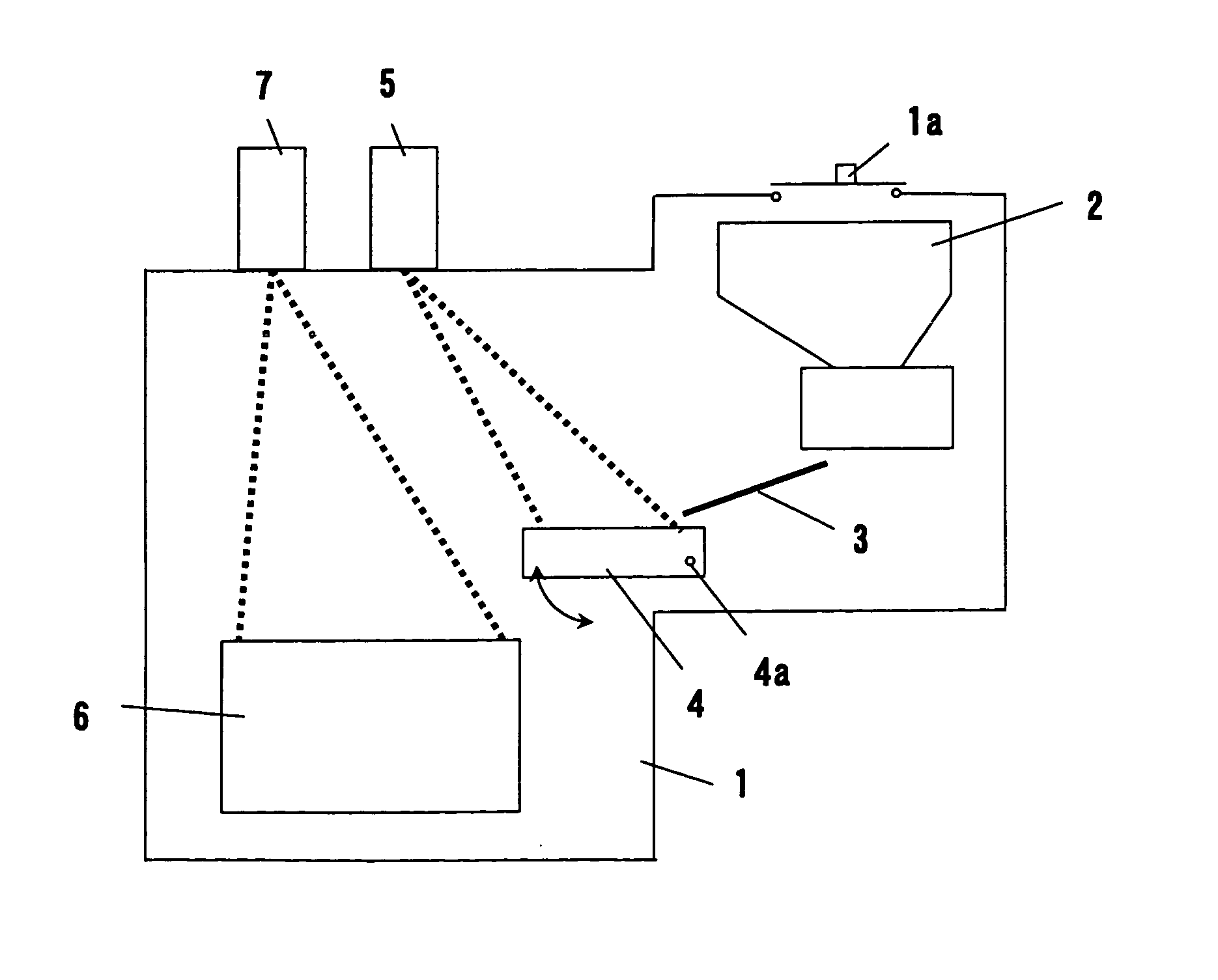
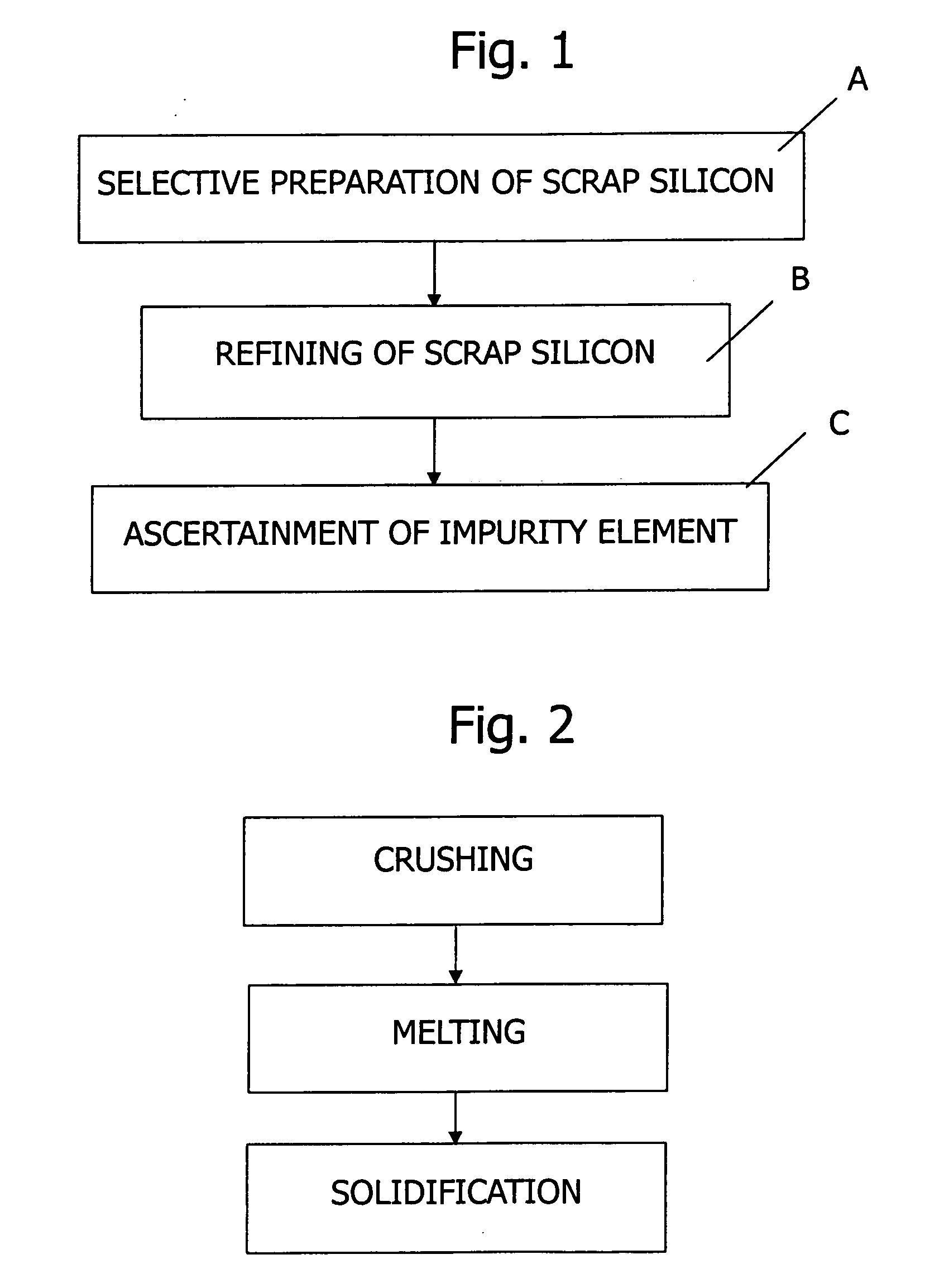
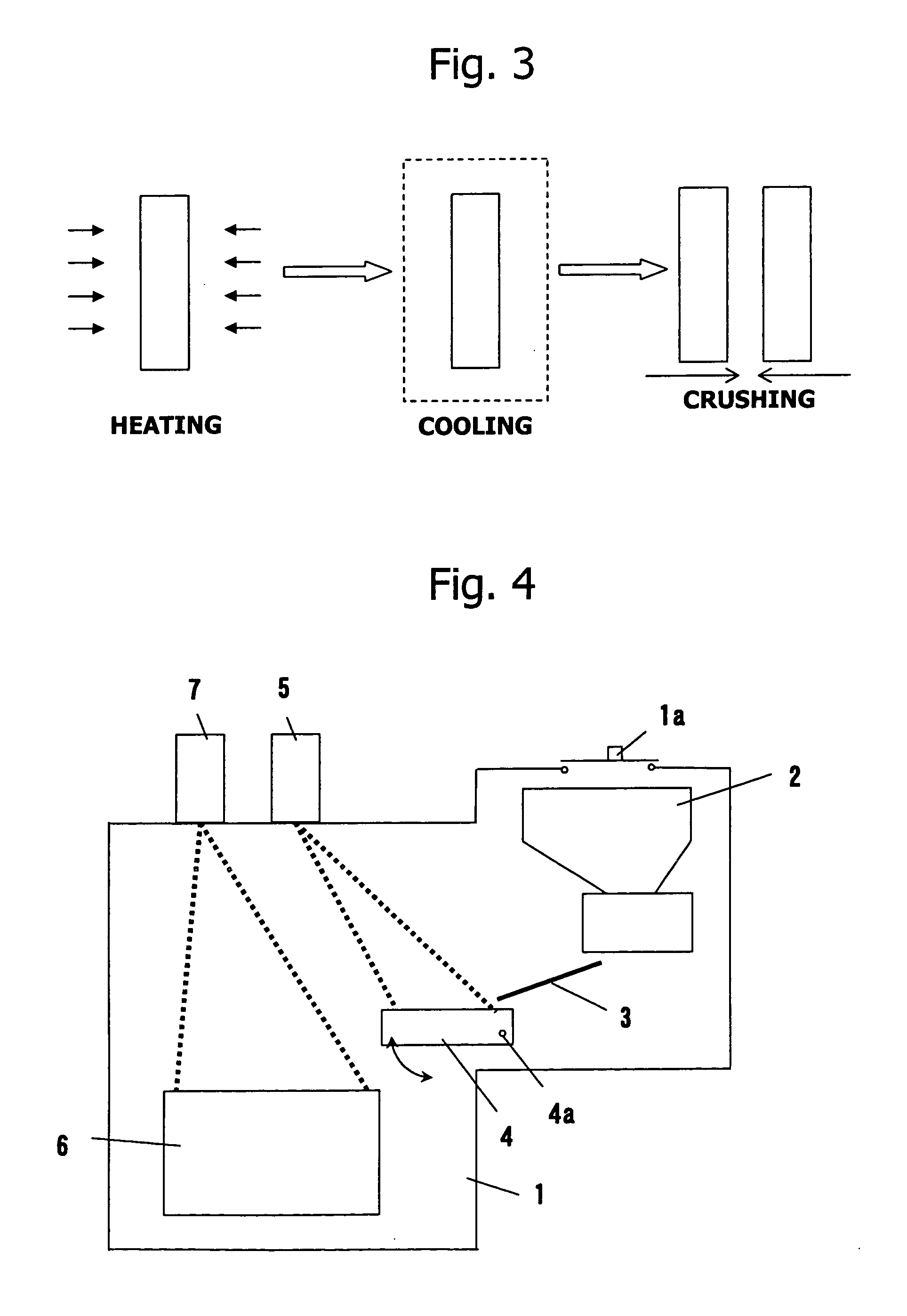
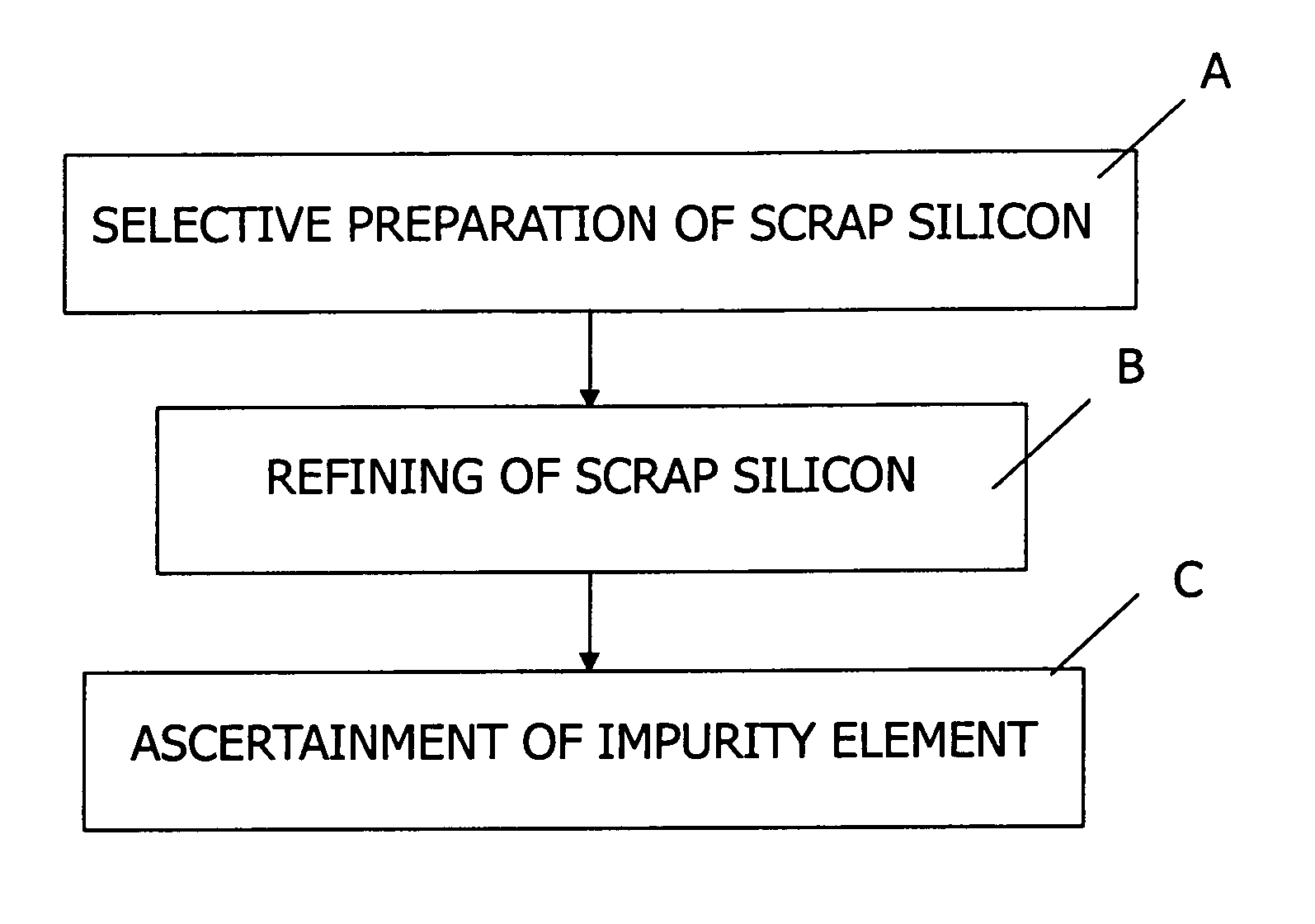
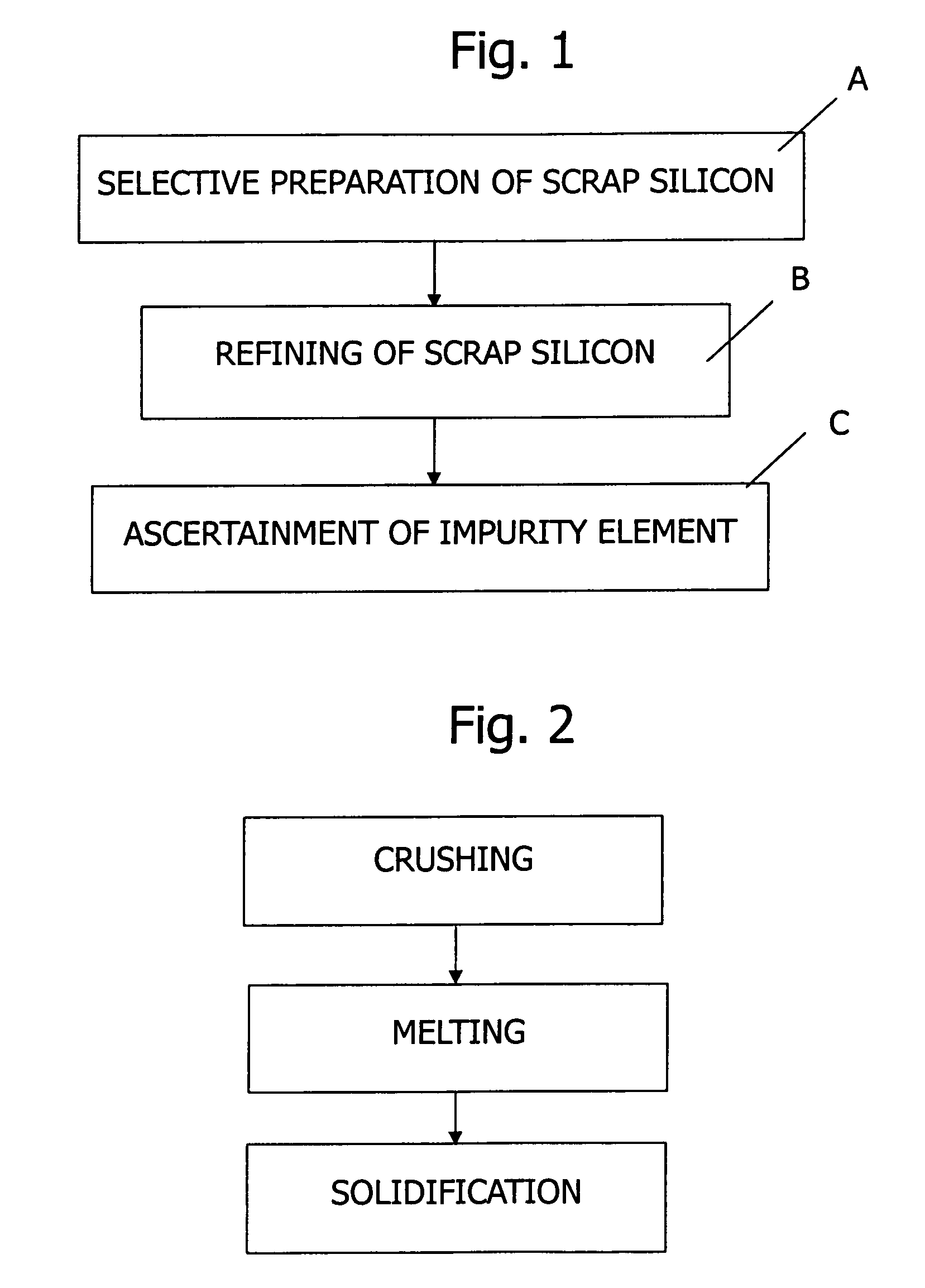
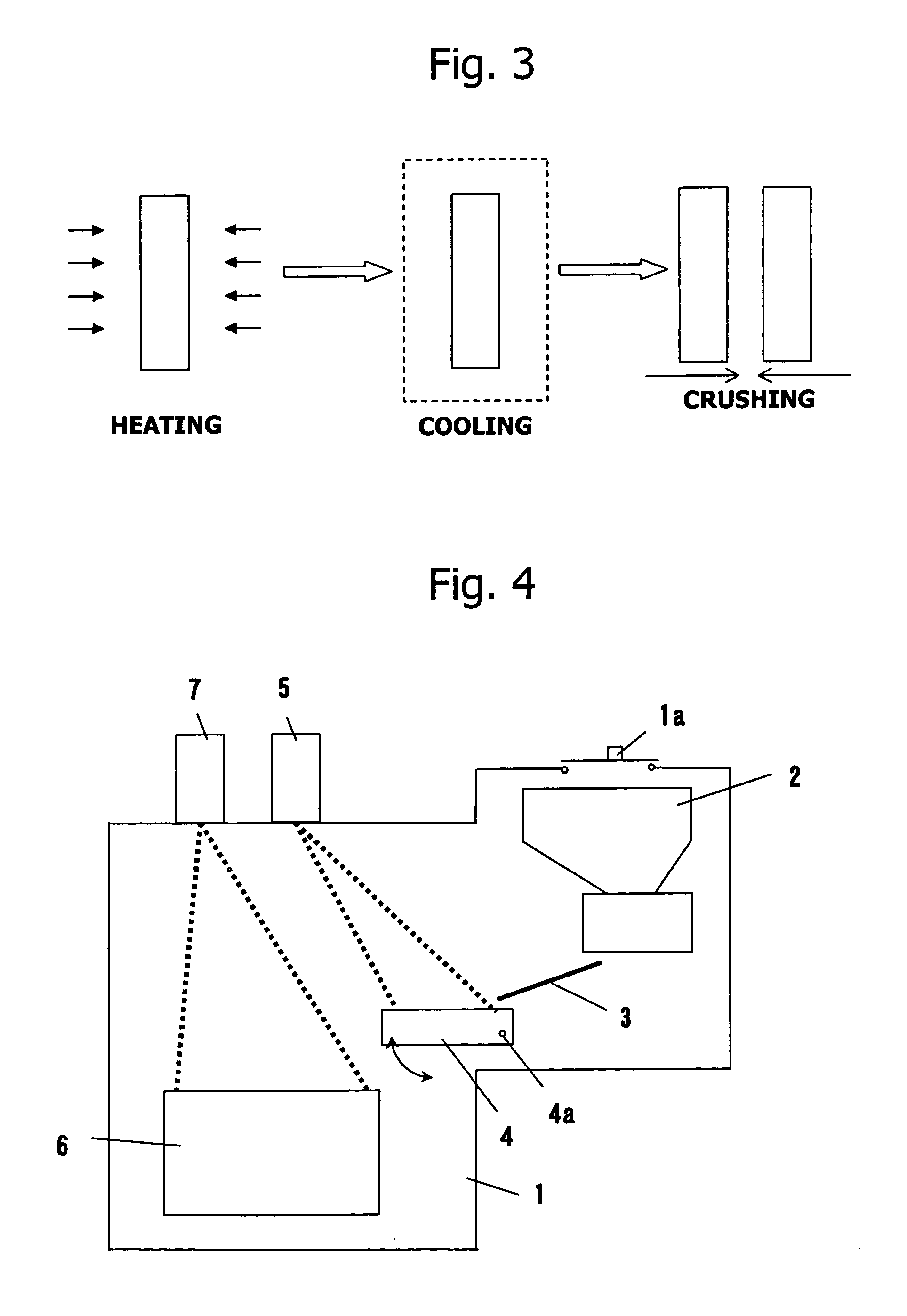
Method of refining scrap silicon using an electron beam
A method of refining scrap silicon using an electron beam includes a step of selectively preparing lumps of n-type scrap silicon containing a specific impurity element as a dopant, a step of crushing the prepared lumps of scrap silicon, a step of placing the crushed silicon into a vacuum vessel, a step of irradiating the crushed silicon which was placed into the vacuum vessel with an electron beam to melt it and vaporize at least a portion of the impurity element, and a step of solidifying the resulting silicon.
Owner:IIS MATERIALS CORP
Preparation method of high-purity LiNH2BH3 and NaNH2BH3
InactiveCN101746728AHigh puritySimple processMonoborane/diborane hydridesMaterials preparationGranularity
The invention belongs to the technical field of material preparation, in particular to a preparation method of high-purity LiNH2BH3 and NaNH2BH3. In the method of the invention, liquid-solid reaction is carried out for synthetizing LiNH2BH3 and NaNH2BH3 under an anhydrous oxygen-free inert atmosphere; after LiNH2BH3 and NaNH2BH3 are washed by solvent, the solvent is removed with a vacuum devolatilization method to obtain high-purity solid LiNH2BH3 and NaNH2BH3; and particles with different granularities are prepared by changing synthesis conditions. The method of the invention has simple technology, convenient operation, easy realization and no high requirements on equipment, can produce high-purity products, and product granularity has certain controllability.
Owner:FUDAN UNIV
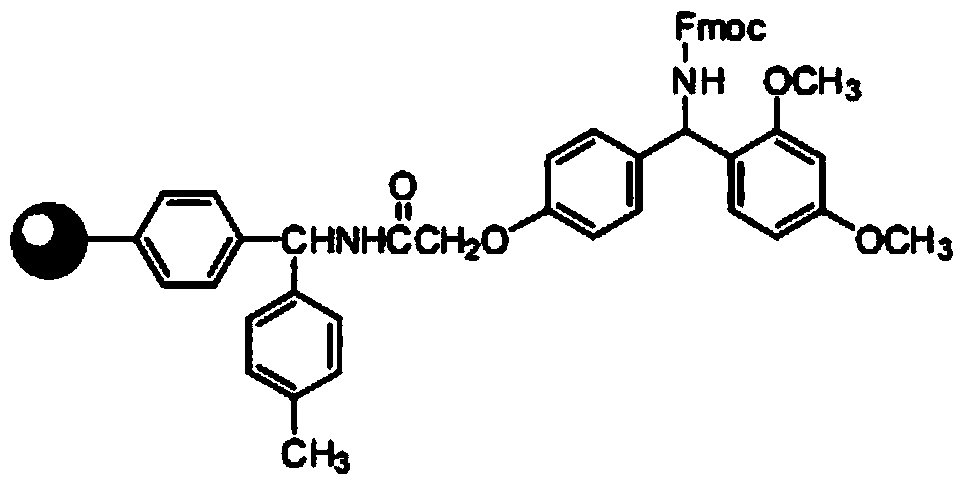
Preparation method of teriparatide
PendingCN109897099AEfficient removalLess prone to side effectsPeptide preparation methodsParathyroid hormonesFreeze-dryingTeriparatide
The invention discloses a preparation method of teriparatide. The preparation method comprises steps as follows: 2-CTC resin is taken as a resin carrier and is coupled with Fmoc-Phe-OH under the action of an activator and a coupling reagent, and Fmoc-Phe-resin is obtained, an Fmoc deprotection reagent is added to the Fmoc-Phe-resin, after a reaction ends, washing is performed with N,N-DMF, Phe-resin is obtained, one-by-one extension coupling is performed on amino acid under the action of a condensation reagent and an activating reagent, and teriparatide-resin is obtained; a lysate containing PhSMe / PhOH / EDT system is added to the teriparatide-resin, and a crude product of teriparatide is obtained; the crude product of teriparatide is separated, purified and freeze-dried by use of a C18 column, and high-purity teriparatide is obtained. The preparation method of teriparatide is low in cost and adopts a simple process. The total yield of teriparatide is increased, and no harm is caused toany environment. The preparation method is suitable for large-scale production.
Owner:哈尔滨吉象隆生物技术有限公司
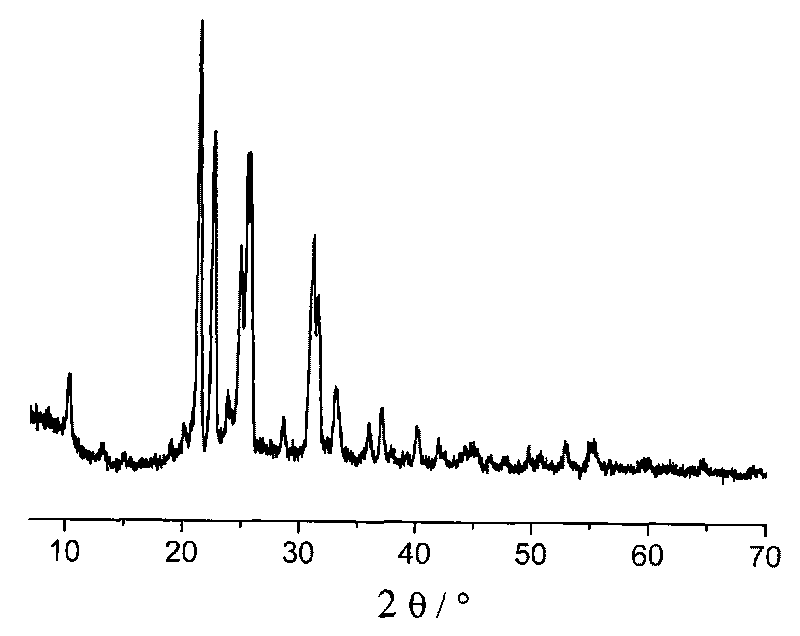
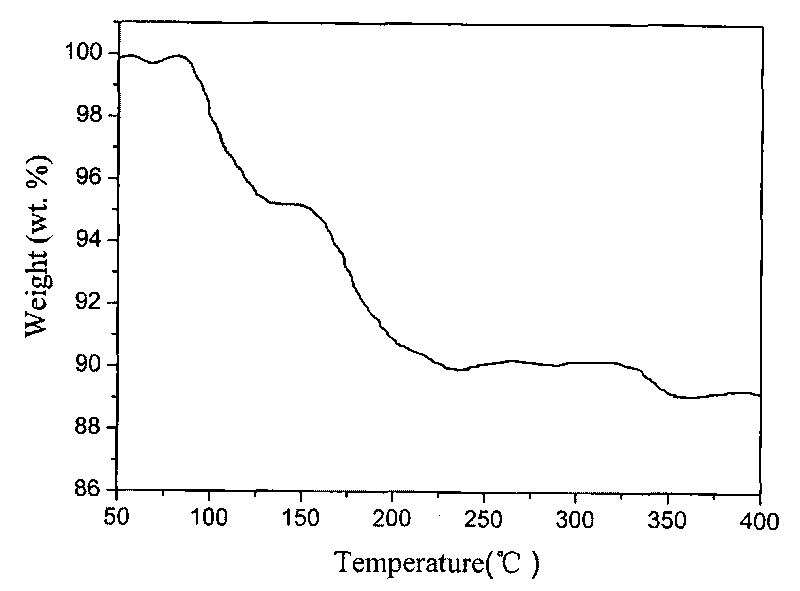
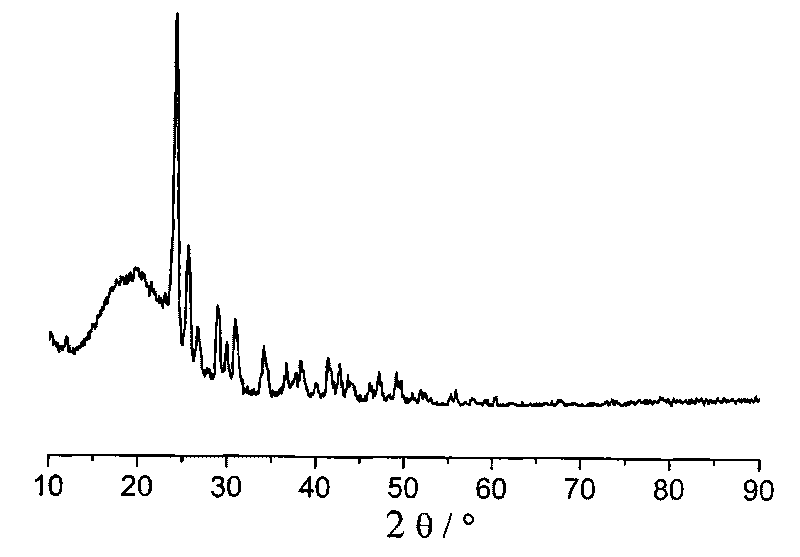
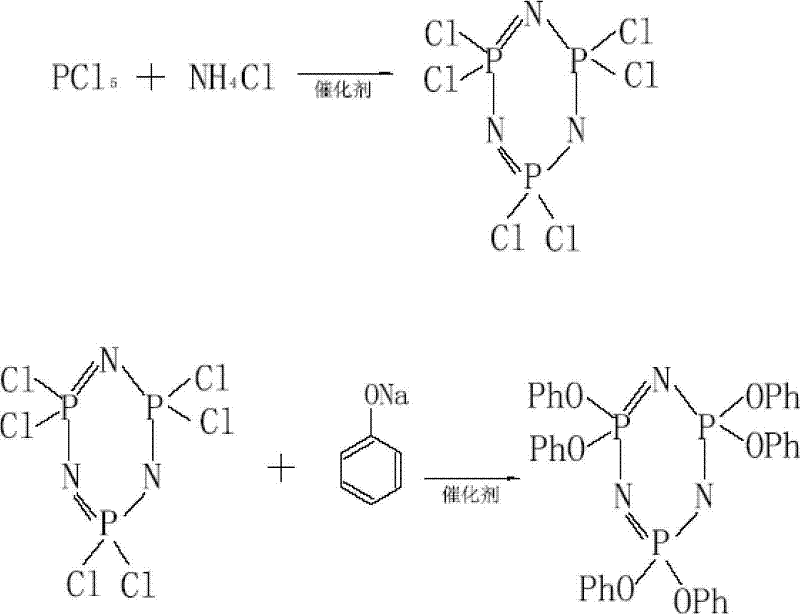
Preparation method of high purity hexaphenoxycyclotriphosphazene
ActiveCN101648978BHigh purityEasy to purifyGroup 5/15 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsSimple Organic CompoundsReaction temperature
The invention relates to a preparation method of hexaphenoxycyclotriphosphazene, in particular to a preparation method of high purity hexaphenoxycyclotriphosphazene, belonging to the technical field of the synthesis of organic compounds with nitrogen and phosphorus and the method contains the reaction process of hexachlorocyclotriphosphazene and sodium phenate substantially. The invention is characterized in that the reaction conditions are as follows: the catalyst is PEG-500-1000, the reaction temperature is 60-140 DEG C, the reaction time is 10-42h, the chlorinity of the target material is less than 500ppm at end point and the reaction solvent is chlorobenzene; concentrative crystallization method is used for purifying, the purity of hexaphenoxycyclotriphosphazene is not less than 99%. The preparation method of the invention has the advantage that the yield is as high as 95%, the type of the used solvent is less, the catalyst can be recycled to use, the consumption of the catalyst is low, the method is simple and is applicable to industrialized production and the purity of the product is high.
Owner:SHANDONG ZESHI NEW MATERIALS TECH CO LTD
A method for synthesizing Ganyrelix
ActiveCN104371010BHigh yieldThe synthesis process is simpleLuteinising hormone-releasing hormonePeptide preparation methodsGanirelixCoupling
The invention relates to the field of medicine synthesis, and discloses a method for synthesizing ganirelix acetate. The method comprises the following steps: carrying out esterification reaction on D-alanine with a protecting group coupled to an N end, and amino resin with a protecting group coupled to amino under the action of a contracting reagent and an activating reagent to obtain peptide resin 1; carrying out gradual extending coupling on other protected amino acids under the action of the contracting reagent and the activating reagent from the peptide resin 1 according to the order from a C end of a ganirelix acetate amino acid sequence to the N end, thus obtaining corresponding peptide resin after extending coupling each time; finally obtaining ganirelix acetate resin, carrying out acidolysis to obtain a ganirelix acetate crude product; and purifying the ganirelix acetate crude product to obtain a ganirelix acetate pure product. According to the method disclosed by the invention, an appropriate synthetic scheme is selected; Ac-D-Nal is selected as the finally synthesized raw material; the whole synthesis process is optimized; an appropriate acidolysis liquid and amino resin are selected to be synthesized; the purity of the ganirelix acetate is improved; and the ganirelix acetate is relatively high in total yield, and is free of harm to any environment.
Owner:CHENGDU SHENGNUO BIOPHARM
A kind of method for preparing sulfuryl fluoride
ActiveCN103864022BSimple processSimple equipmentSulfur-halogen-hydrogen-oxygen compoundsSulfuryl fluorideFluoridation reaction
The invention provides a sulfuryl fluoride preparation method using sulfuryl chloride as a raw material by two-stage fluorination reaction, the sulfuryl fluoride preparation method has the advantages of simple technology and equipment, easy enlarging production, high product purity and the like, and prepared sulfuryl fluoride can be used as a fumigant to replace methyl bromide.
Owner:SINOCHEM LANTIAN +1
D-A type excited state proton transfer high-efficiency fluorescent material, and preparation method and application thereof
ActiveCN111057008AEasy to synthesizeEasy to purifyOrganic chemistrySolid-state devicesQuantum efficiencyBoronic acid
The invention belongs to the technical field of organic fluorescent materials, and discloses a D-A type excited state proton transfer high-efficiency fluorescent material, and a preparation method andan application thereof. The specific structure of the D-A type excited state proton transfer high-efficiency fluorescent material is shown in the specification. The method comprises the following steps: carrying out a Suzuki coupling reaction on triphenylamine-4-boronic acid pinacol ester and 4-bromo-2-((N-phenyl)-9,10-phenanthroimidazole)phenol in a catalytic system, and carrying out subsequenttreatment to obtain the organic small molecular fluorescent material with the D-A structure. The fluorescent material has efficient fluorescence quantum efficiency, and can improve the luminous efficiency of an OLED device; and the large delta ET2-T1 and the small delta ES1-T2 are helpful for T2 to jump to the reverse system of S1, so that the exciton utilization rate and the external quantum efficiency of the OLED device are improved. The method is simple. The application is an application f the fluorescent material in the organic light-emitting device.
Owner:SOUTH CHINA UNIV OF TECH
Method for synthesizing ganirelix acetate
ActiveCN104371010AHigh overall yieldHigh yieldLuteinising hormone-releasing hormonePeptide preparation methodsPeptideAmino acid
The invention relates to the field of medicine synthesis, and discloses a method for synthesizing ganirelix acetate. The method comprises the following steps: carrying out esterification reaction on D-alanine with a protecting group coupled to an N end, and amino resin with a protecting group coupled to amino under the action of a contracting reagent and an activating reagent to obtain peptide resin 1; carrying out gradual extending coupling on other protected amino acids under the action of the contracting reagent and the activating reagent from the peptide resin 1 according to the order from a C end of a ganirelix acetate amino acid sequence to the N end, thus obtaining corresponding peptide resin after extending coupling each time; finally obtaining ganirelix acetate resin, carrying out acidolysis to obtain a ganirelix acetate crude product; and purifying the ganirelix acetate crude product to obtain a ganirelix acetate pure product. According to the method disclosed by the invention, an appropriate synthetic scheme is selected; Ac-D-Nal is selected as the finally synthesized raw material; the whole synthesis process is optimized; an appropriate acidolysis liquid and amino resin are selected to be synthesized; the purity of the ganirelix acetate is improved; and the ganirelix acetate is relatively high in total yield, and is free of harm to any environment.
Owner:CHENGDU SHENGNUO BIOPHARM
Method of refining scrap silicon using an electron beam
InactiveUS7632329B2Easy to disassembleEfficient preparationElectric furnaceSilicon compoundsDopantImpurity
A method of refining scrap silicon using an electron beam includes a step of selectively preparing lumps of n-type scrap silicon containing a specific impurity element as a dopant, a step of crushing the prepared lumps of scrap silicon, a step of placing the crushed silicon into a vacuum vessel, a step of irradiating the crushed silicon which was placed into the vacuum vessel with an electron beam to melt it and vaporize at least a portion of the impurity element, and a step of solidifying the resulting silicon.
Owner:IIS MATERIALS CORP
A self-assembled rod-shaped manganese-zinc ferrite magnetic material and preparation method thereof
The invention provides a manganese-zinc ferrite magnetic material with uniform particle size, regular particle morphology, and good dispersion. The material is a self-assembled submicron-level rod assembled by octahedral units, and has a structural formula of MnxZn1-xFe2O4, wherein x is no smaller than 0 and no greater than 1. In a reactant, a molar ratio of the elements are that (Mn+Zn) / Fe=1:2. Reaction medium mass / reactant total mass=0-6:1. The invention also provides a process for preparing the material. In the reaction process of the method, molten salt exists among produced manganese-zinc ferrite particles, such that particle agglomeration can be prevented. With the method provided by the invention, stoichiometric ratio of the components can be precisely controlled. The method also has the advantages of simple process, high-purity product, controllable particle morphology, no need of mechanical ball milling, no doping, narrow particle size distribution, wide raw material source, and the like.
Owner:CHINA UNIV OF GEOSCIENCES (WUHAN)
A method for synthesizing teriparatide
ActiveCN104910269BHigh yieldNo harmPeptide preparation methodsParathyroid hormonesAcid hydrolysisCombinatorial chemistry
Owner:CHENGDU SHENGNUO BIOTEC CO LTD
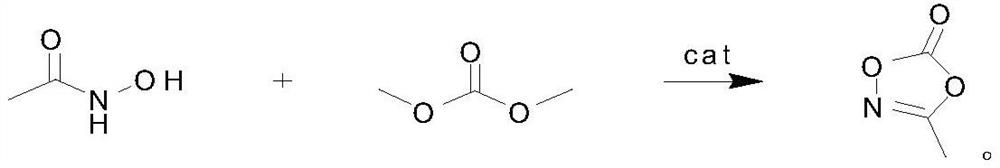
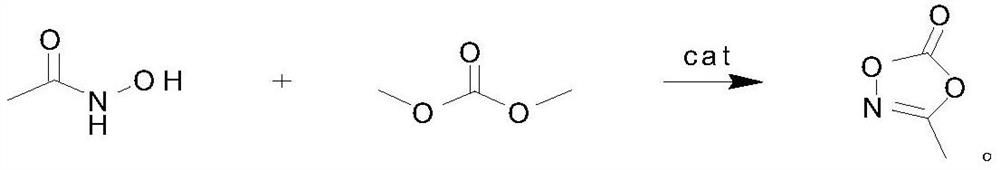
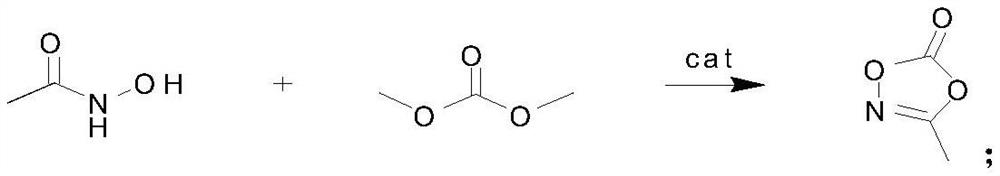
Preparation method of 3-methyl-1, 4, 2-dioxazole-5-one, product and application
ActiveCN111689926AReduce post-purification stepsHigh purityOrganic chemistrySecondary cellsTrans esterificationPtru catalyst
The invention discloses a preparation method of 3-methyl-1, 4, 2-dioxazole-5-one, a product and application. The method is characterized by comprising the following steps of: adding acetohydroxamic acid, dimethyl carbonate and a catalyst into a reaction flask, performing heating to 110DEG C, carrying out reaction while fractionating a byproduct methanol, carrying out the reaction for 4h, then carrying out reduced pressure distillation on the reaction liquid, and collecting 89DEG C / 10mmHg fraction to obtain 3-methyl-1, 4, 2-dioxazole-5-one. According to the method, the 3-methyl-1, 4, 2-dioxazole-5-one is synthesized by using an ester exchange method, few byproducts are produced, the catalyst can be separated after being filtered, and the later purification step is greatly reduced, so that ahigh-purity product is easy to obtain.
Owner:ZHANGJIAGANG HUASHENG CHEM CO LTD
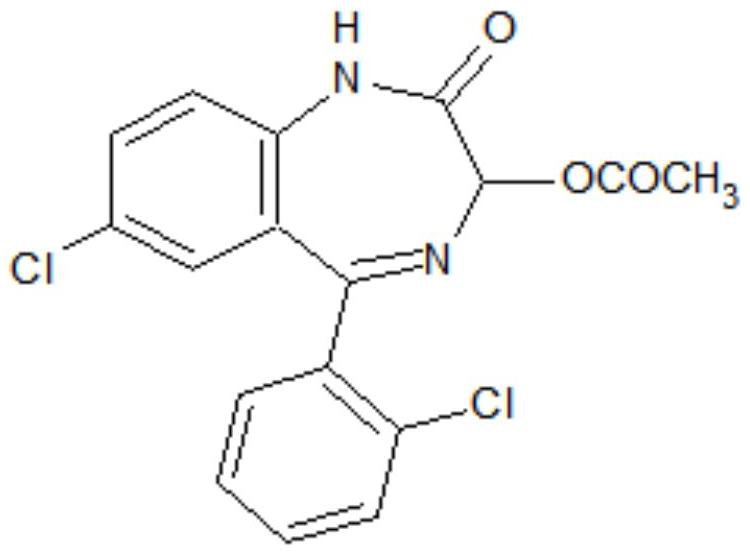
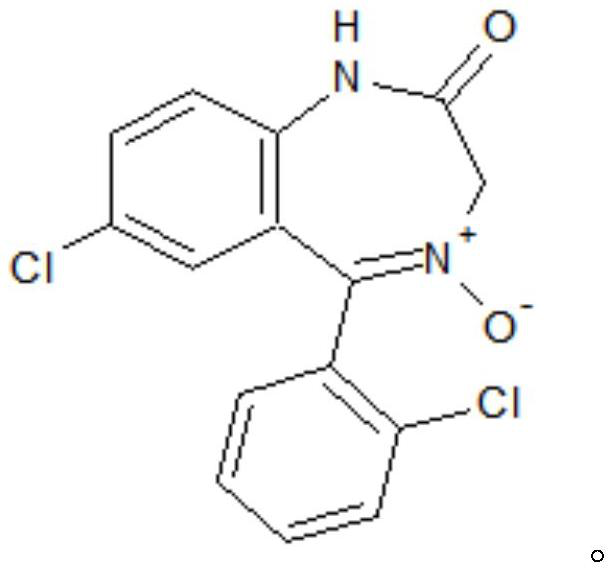
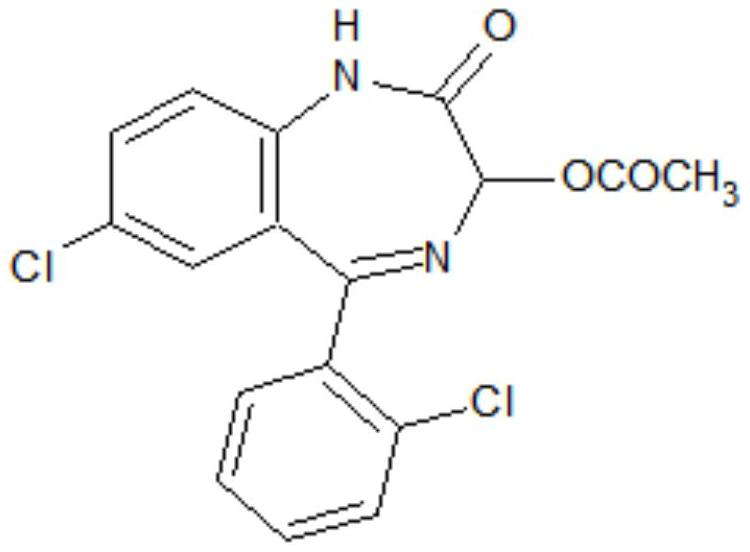
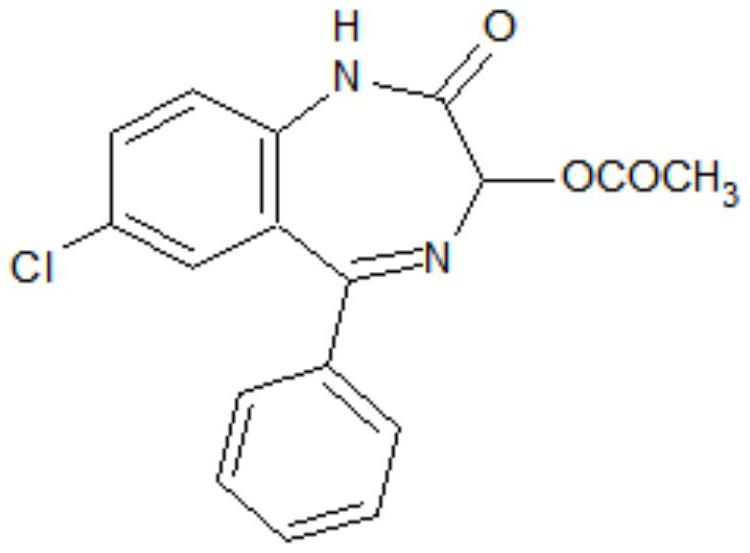
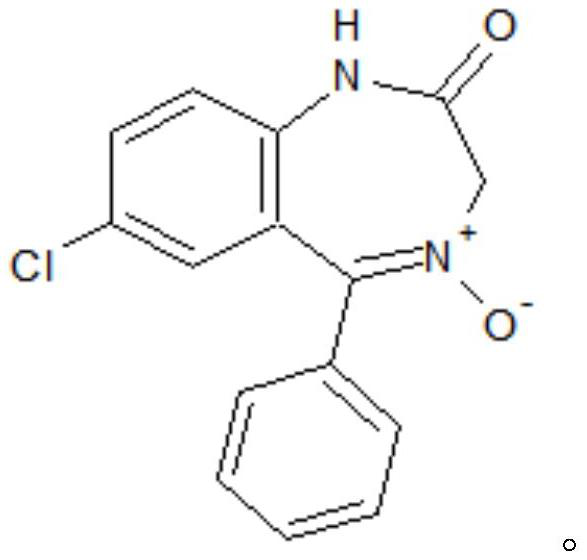
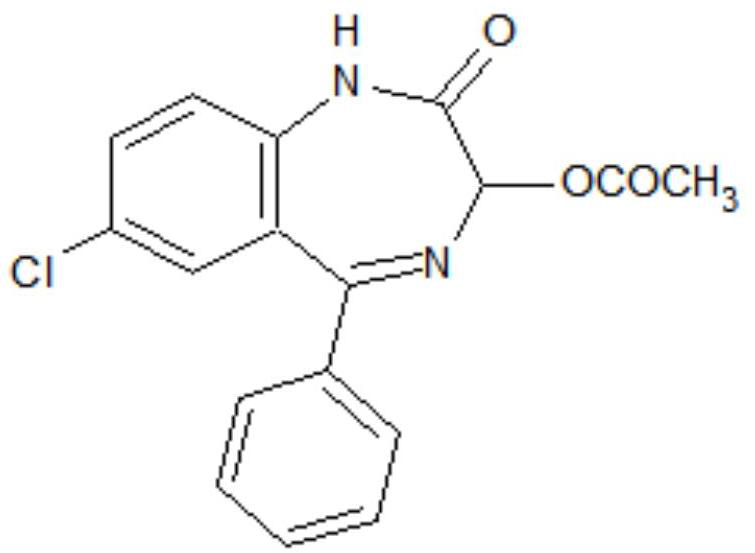
Preparation method of lorazepam intermediate
The invention discloses a preparation method of a lorazepam intermediate, which comprises the following steps: adding 4-dimethylaminopyridine into a mixed solution of 7-chloro-2-oxo-5-(2-chlorphenyl)-1, 4-benzodiazepin-4-oxide, acetic anhydride and an aprotic polar solvent, stirring, heating, carrying out heat preservation reaction, cooling after the reaction is finished, adding water to separate out materials, filtering, washing with water, refining to obtain a target product. According to the invention, under the condition of greatly reducing the dosage of acetic anhydride, the yield of acylation and rearrangement reactions of lorazepam is ensured, and the discharge of acid-containing wastewater is greatly reduced; in addition, in the reaction process, the temperature is stable, the phenomenon of violent rising does not occur, and the production safety is greatly improved.
Owner:HUAZHONG PHARMA
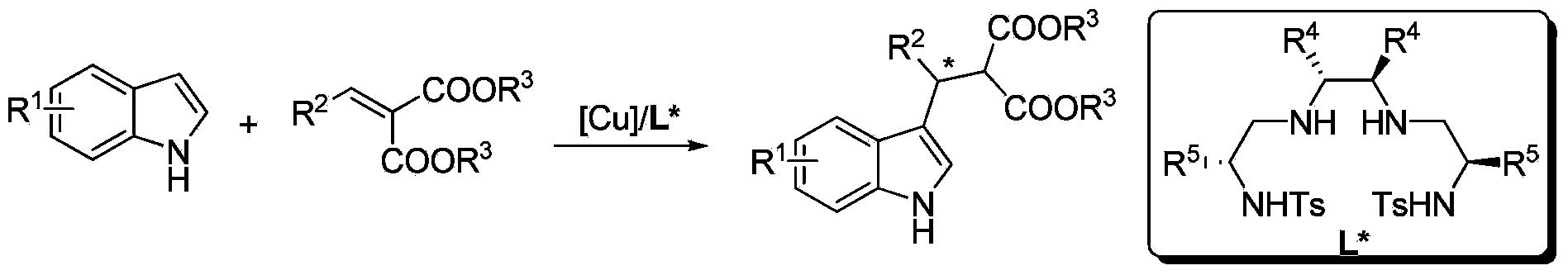
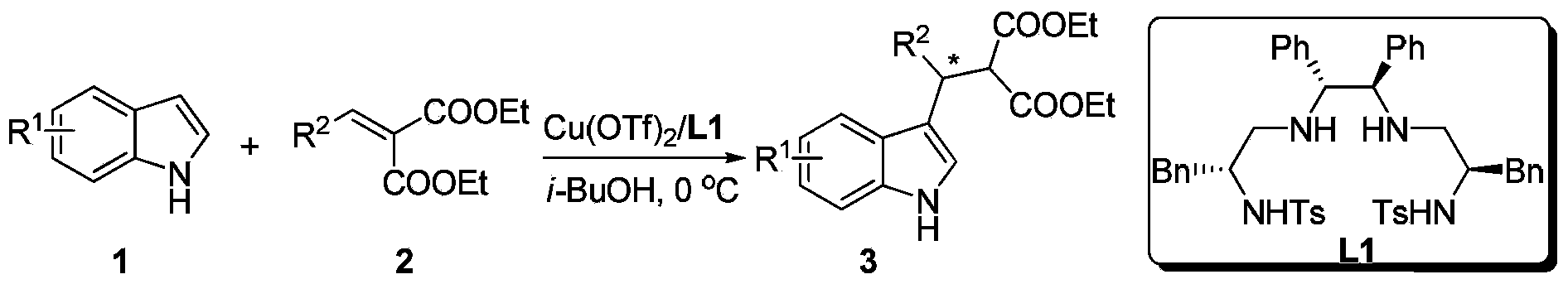
Method for using Cu to catalyze indole to perform asymmetric Friedel-Crafts acylation reaction
InactiveCN104119262ARaw materials are easy to getEasy to operateOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsIsobutanolMalonate
A method for using Cu to catalyze indole to perform asymmetric Friedel-Crafts acylation reaction is disclosed. Employed reaction substrates comprise an indole and a (phenylmethylene)malonate, and a catalysis system is a Cu(II)-disulfonamide diamine ligand complex. The reaction is performed under the following conditions that the temperature is in the scope of room temperature to -20 DEG C, the solvent is ethanol, methanol, isobutanol, n-propanol and other alcohol solvents, the catalytic amount is 1-10%, an employed metal precursor is copper(II) trifluoromethanesulfonate (Cu(OTf)2) or copper perchlorate (Cu(ClO4)2.6H2O), and an employed chiral ligand is a disulfonamide diamine ligand. A catalyst preparation method comprises under the protection of N2, stirring the copper metal precursor and the chiral disulfonamide diamine ligand in the alcohol solveny at room temperature for 2 hours, so as to obtain the catalyst. Asymmetric Friedel-Crafts acylation reaction can be smoothly performed on various substituted indoles and a (phenylmethylene)malonate for obtaining a corresponding chiral product. The yield is up to 99%, and the enantiomeric excess value is up to 96%.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Process for production of iodine compounds and process for production of high-purity 5-iodo-2-methylbenzoic acid
InactiveCN100436385CHigh purity productHigh purityCarboxylic acid nitrile preparationMolecular sieve catalystsOrganic solventChemical products
Provided is a production method for an iodine compound in which iodine is reacted with a substrate in the presence of a porous material having a pore diameter of 500 nm or less or in the presence of the above porous material and an oxidizing agent and a production process for high purity 5-iodo-2-methylbenzoic acid comprising an iodination reaction step carried out by the above-mentioned, a crystal precipitation and separation step in which a product is precipitated by adding water or cooling and then separated and a purification step in which crystal separated is recrystallized using an organic solvent. According to the production method for an iodine compound described above, iodine can be introduced into various substrates at a high selectivity. Since expensive metals and specific reagents do not have to be used, it can readily be carried out in an industrially scale, and the product having a high purity can be obtained. Further, the process comprising the iodination reaction, separation and purification steps described above makes it possible to readily obtain at a high yield, 5-iodo-2-methylbenzoic acid having a high purity which is useful in uses for functional chemical products such as medicines. The process of the present invention comprising iodination reaction, separation and purification steps is characterized by that it is simple in terms of a procedure and that the purification load is smaller, and it is very advantageous in industrially carrying out.
Owner:日本精细化工株式会社
Apparatus and method for cell kill confirmation
ActiveUS20220348865A1Improve on and overcome deficiencyHigh purity productMicroscopesIndividual particle analysisErbium lasersFluorochrome Dye
Owner:ABS GLOBAL
Preparation method of oxazepam intermediate
PendingCN113816913AEmission reductionLow costOrganic chemistryAcetic anhydrideCombinatorial chemistry
The invention discloses a preparation method of an oxazepam intermediate, which comprises the following steps: adding acetic anhydride and anhydrous acetate into 7-chloro-2-oxo-5-phenyl-2, 3-dihydro-1-H-1, 4-benzodiazepine-4-oxide in an aprotic polar solvent, stirring and heating to carry out heat preservation reaction, and then cooling, separating and refining to obtain the oxazepam intermediate as shown in a formula I. according to the method, acylation and rearrangement are carried out under a formed homogeneous system, so that the reaction yield is increased, the acetic anhydride consumption is greatly reduced, and the raw material cost and the sewage treatment cost are reduced; in addition, the reaction is stable in a homogeneous system, the safety is obviously improved, and the method is suitable for large-scale industrial production.
Owner:HUAZHONG PHARMA
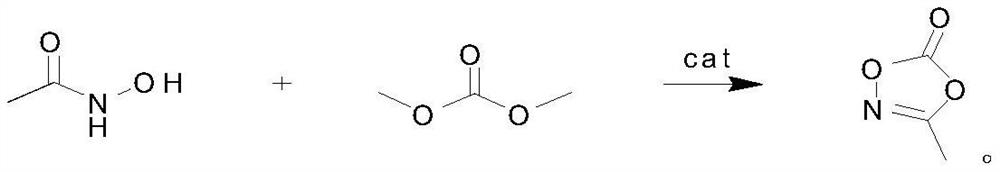
Preparation method, product and application of 3-methyl-1,4,2-dioxazol-5-one
ActiveCN111689926BReduce post-purification stepsHigh purityOrganic chemistrySecondary cellsPtru catalystKetone
The invention discloses a preparation method, product and application of 3-methyl-1,4,2-dioxazol-5-ketone, which is characterized in that it is carried out according to the following steps: acetohydroxamic acid, dimethyl carbonate and catalyst into the reaction flask, and then heated to 110°C, and the by-product methanol was fractionated while reacting. After 4 hours of reaction, the reaction liquid was subjected to vacuum distillation, and the fraction at 89°C / 10mmHg was collected to obtain 3-methyl ‑1,4,2‑Dioxazol‑5‑one. The present invention uses the transesterification method to synthesize 3-methyl-1,4,2-dioxazol-5-ketone, with few by-products, and the catalyst can be separated by filtration, which greatly reduces the later purification steps, thereby easily obtaining high-purity products .
Owner:ZHANGJIAGANG HUASHENG CHEM CO LTD
Bifunctional ionic liquid and application thereof
PendingCN112430214AThe synthesis method is simpleImprove anti-wear and anti-friction performanceOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystBifunctional
The invention provides a bifunctional ionic liquid and an application thereof, and relates to the technical field of lubricating oil, the bifunctional ionic liquid is obtained by reaction of ionic liquid 1-alkyl-3-methylimidazole bromine and bis (2-ethylhexyl) hydrogen phosphate, and the bifunctional ionic liquid is applied to synthetic ester lubricating oil as a catalyst and a lubricating oil additive. The bifunctional ionic liquid is applied to synthesis of ester lubricating oil as the catalyst, separation is not needed, and the performance of the ester lubricating oil can be improved.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI +1
A kind of immobilized ionic liquid catalyst and its application in the preparation of synthetic ester lubricating oil
ActiveCN105879907BThe synthesis method is simpleHigh activityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAlcoholMesoporous silica
The invention discloses a solid-supported ionic liquid catalyst. The catalyst is prepared by the following method: 1) Stir ionic liquid, absolute ethanol, and tetraethyl orthosilicate at a constant temperature of 40oC~70oC until the ionic liquid is dissolved and the solution is clear. , then add hydrochloric acid solution, continue stirring for 1 h to 3 h, and then let it stand for 12 h to 24 h to obtain the initial product of the mesoporous silica solid-supported ionic liquid catalyst; 2) The mesoporous silica solid-supported ionic liquid catalyst is obtained The initial product of the silicon solid-supported ionic liquid catalyst is dried under vacuum at 80oC~100oC for 3 h~6 h, and the mesoporous silica solid-supported ionic liquid catalyst is obtained. The invention also discloses the application of the catalyst in the preparation of synthetic ester lubricating oil. The catalyst synthesis method involved in the present invention is simple and has high catalyst activity, which greatly accelerates the process of its industrial application.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com