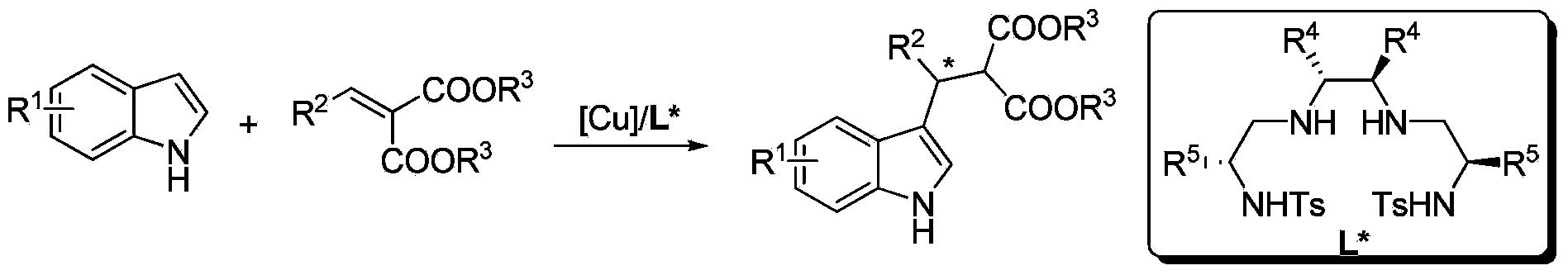
Method for using Cu to catalyze indole to perform asymmetric Friedel-Crafts acylation reaction
A Friedel-Crafts alkylation and asymmetric technology, applied in the field of Cu-catalyzed asymmetric Friedel-Crafts alkylation of indole, to achieve the effects of simple synthesis, easy availability of raw materials, and simple synthesis of chiral ligands
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
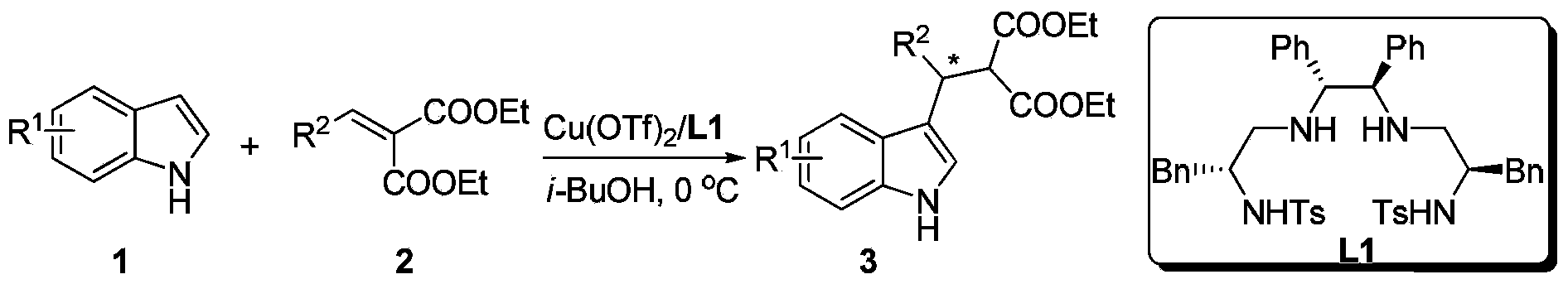
[0026] Example 1: Synthesis of indole derivatives 3 by Cu-catalyzed asymmetric Friedel-Crafts alkylation reaction
[0027] Put copper salt metal precursors into the reaction bottle: copper trifluoromethanesulfonate (0.0125 mmol) and chiral ligand L1 (0.0125 mmol), add 1 ml of i-BuOH solvent after nitrogen replacement, and stir at room temperature for 2 h , placed in a cold bath at 0°C, added substrate 2 (74.5 mg, 0.3 mmol) and stirred for 10 minutes, then added substrate 1 (29.3 mg, 0.25 mmol), reacted at room temperature, and TLC detected that substrate 1 was completely The disappearance means that the reaction is complete, and the reaction is about 2-12 hours, and the reaction solvent is drained with an oil pump. Then, column chromatographic separation afforded the pure product. The reaction formula is as follows:
[0028]
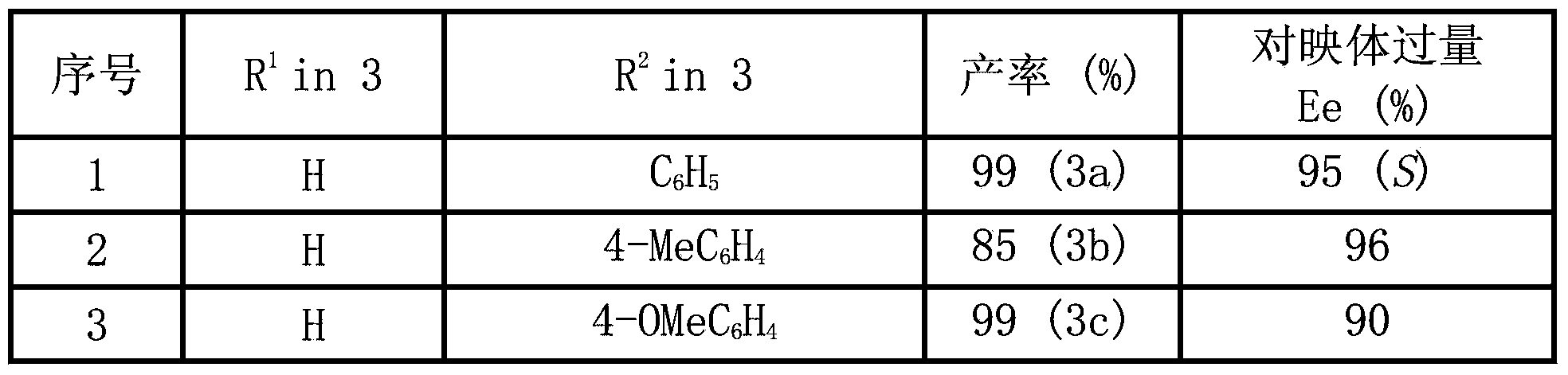
[0029](S)-Ethyl2-ethoxycarbonyl-3-(3-indolyl)-3-phenyl propanoate(3a).99%yield,95%ee,[α] 20 D =+63.4(c1.0,CH 2 Cl 2 ); 1 H NMR (400MHz, CDCl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com