Bifunctional ionic liquid and application thereof
An ionic liquid, dual-function technology, applied in the direction of base materials, additives, organic compounds/hydrides/coordination complex catalysts, etc. The effect of the simple method of synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Weigh 4.3824g of ionic liquid 1-butyl-3-methylimidazolium bromide, and simultaneously weigh 7.0932g of bis(2-ethylhexyl) hydrogen phosphate in a 250mL round bottom flask, then add 100ml of di Methyl chloride and 5ml of water were stirred evenly at room temperature and marked as a solution.
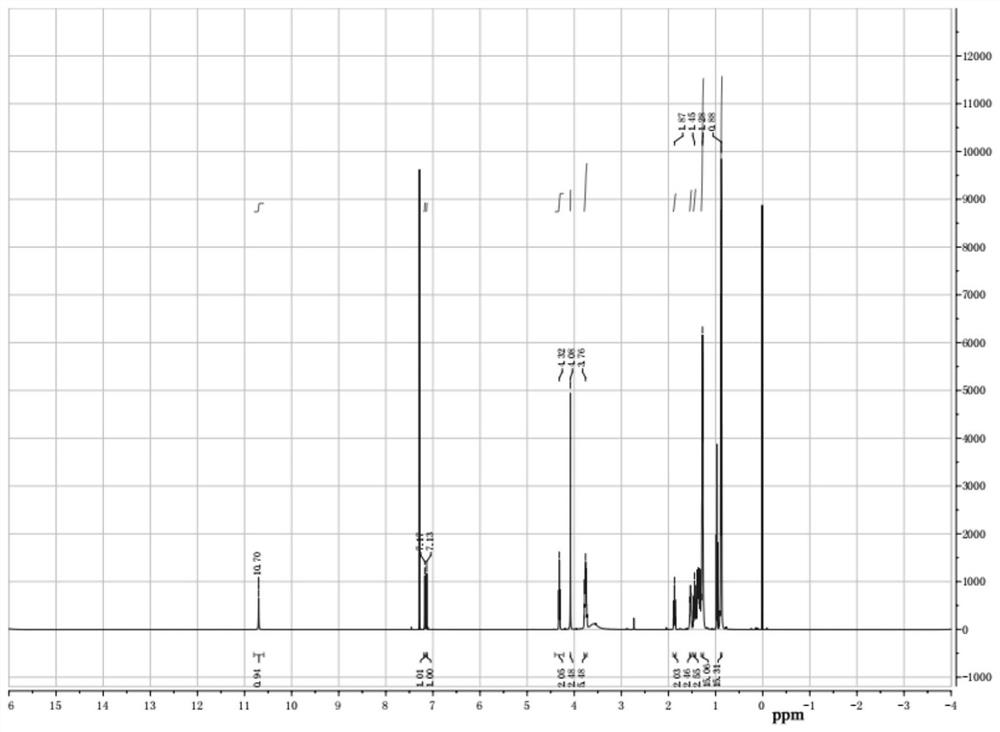
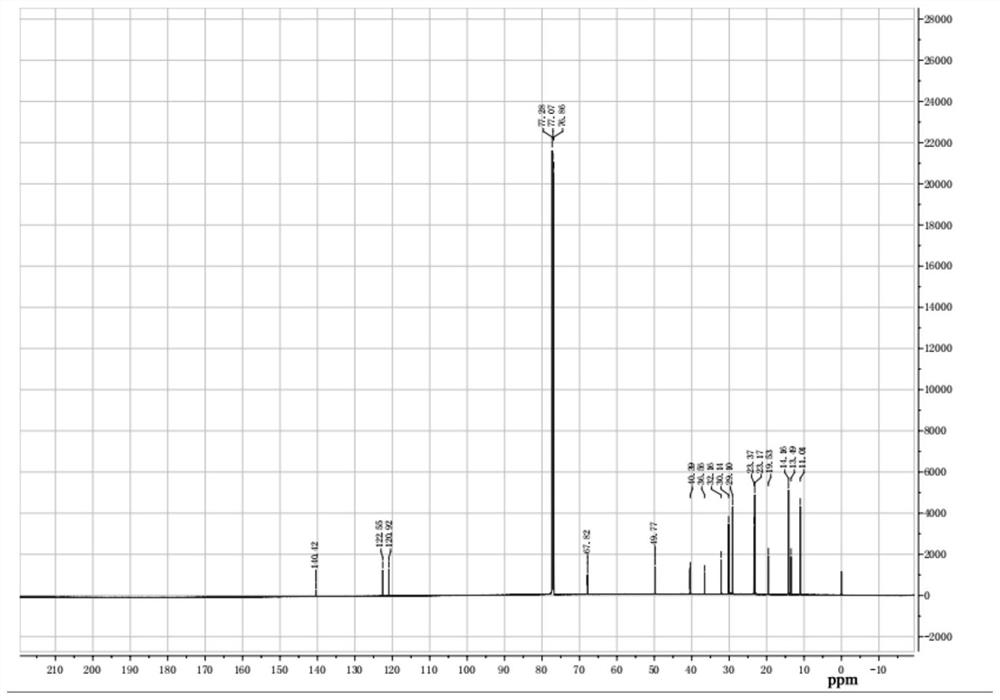
[0034] (2) configuration mass fraction is the sodium hydroxide aqueous solution of 30%, is marked as b solution, b solution is added dropwise in a solution, room temperature continues to stir for 2 hours after dropwise addition, after reaction finishes, pour into the separating funnel statically Place it for a period of time and wash the filtrate several times with distilled water, then use a rotary evaporator to remove water and dichloromethane, and finally dry it under vacuum at 70 degrees Celsius to obtain bifunctional ionic liquid Bi-IL4. For its nuclear magnetic resonance spectrum, please refer to the attached figure 1 And attached figure 2 .
Embodiment 2
[0036] (1) Weigh 4.9436g of ionic liquid 1-hexyl-3-methylimidazolium bromide, and simultaneously weigh 7.0932g of bis(2-ethylhexyl) hydrogen phosphate in a 250mL round bottom flask, then add 100ml of dichloro Methane and 5ml of water were stirred evenly at room temperature and marked as a solution.
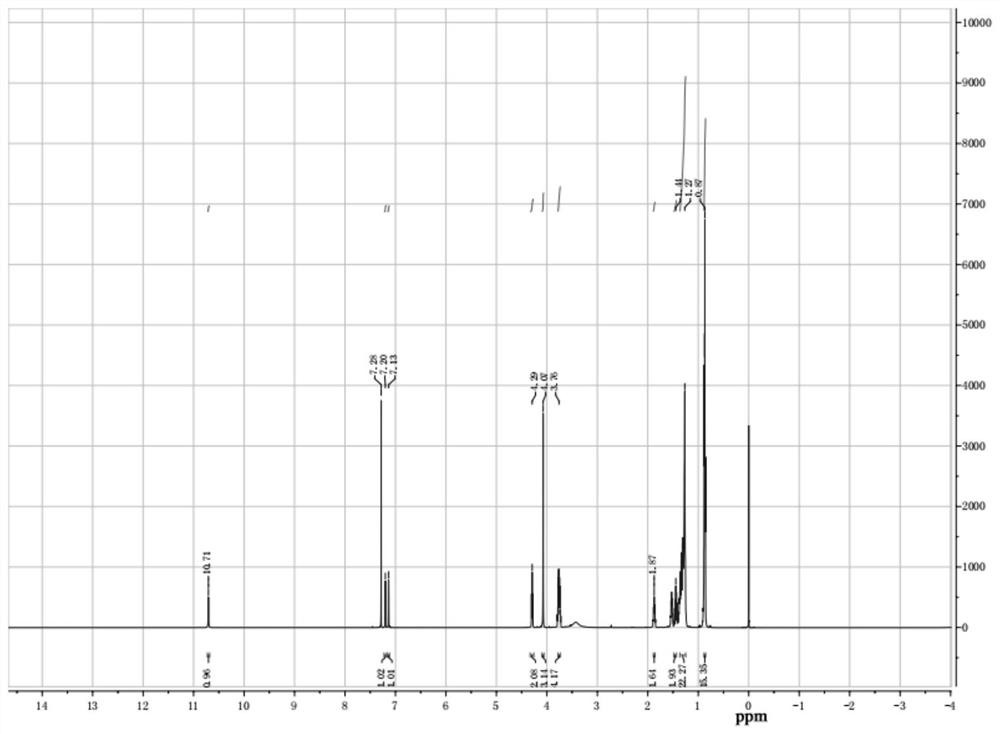
[0037] (2) configuration mass fraction is the sodium hydroxide aqueous solution of 30%, is marked as b solution, b solution is added dropwise in a solution, room temperature continues to stir for 2 hours after dropwise addition, after reaction finishes, pour into the separating funnel statically Place it for a period of time and wash the filtrate repeatedly with distilled water for several times, then use a rotary evaporator to remove water and dichloromethane, and finally dry it under vacuum at 70 degrees Celsius to obtain bifunctional ionic liquid Bi-IL6. The nuclear magnetic resonance spectrum is shown in image 3 , 4.
Embodiment 3
[0039](1) Weigh 5.5046g of ionic liquid 1-octyl-3-methylimidazolium bromide, and simultaneously weigh 7.0932g of bis(2-ethylhexyl) hydrogen phosphate in a 250mL round bottom flask, then add 100ml of di Methyl chloride and 5ml of water were stirred evenly at room temperature and marked as a solution.
[0040] (2) configuration mass fraction is the sodium hydroxide aqueous solution of 30%, is marked as b solution, b solution is added dropwise in a solution, room temperature continues to stir for 2 hours after dropwise addition, after reaction finishes, pour into the separating funnel statically Place it for a period of time and wash the filtrate repeatedly with distilled water for several times, then use a rotary evaporator to remove water and dichloromethane, and finally dry it under vacuum at 70 degrees Celsius to obtain bifunctional ionic liquid Bi-IL8. The nuclear magnetic resonance spectrum is shown in Figure 5 , 6.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com