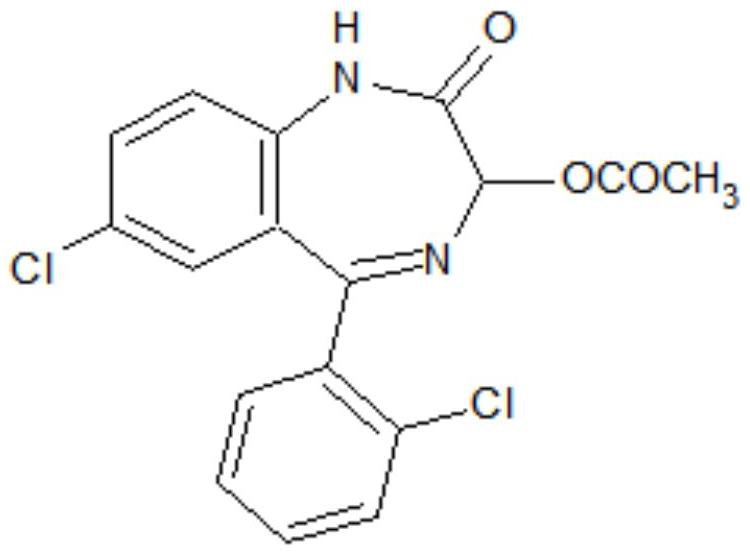
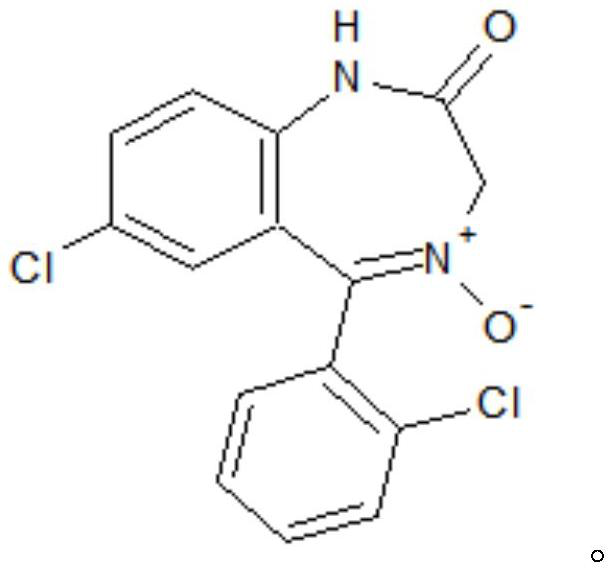
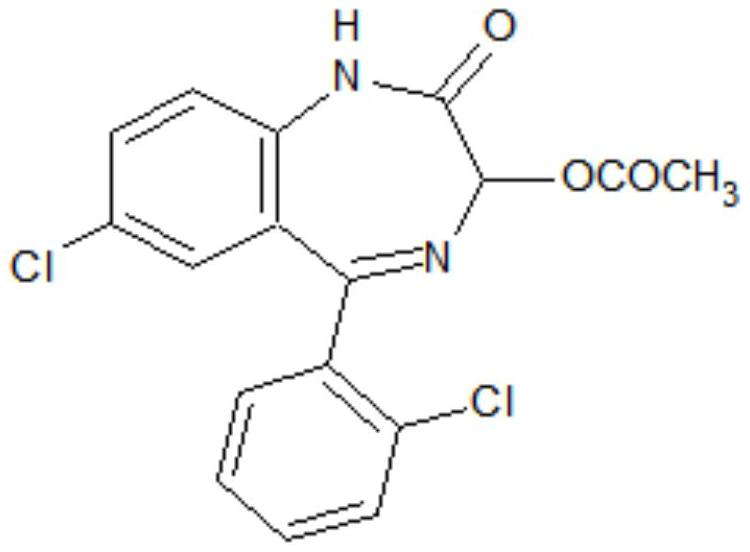
Preparation method of lorazepam intermediate
A technology of intermediates and compounds, applied in the field of preparation of pharmaceutical intermediates, can solve the problems of increasing sewage treatment costs, increasing the difficulty of temperature control, increasing organic impurities, etc., reducing raw material costs and sewage treatment costs, and improving production safety performance, emission reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] Above-mentioned preparation method comprises the following steps:
[0035] S1. To 7-chloro-2-oxo-5-(2-chlorophenyl)-1,4-benzodiazepine-4-oxide (hereinafter referred to as formula II compound), acetic anhydride, aprotic Add 4-dimethylaminopyridine to the polar solvent, stir and raise the temperature to 85°C-90°C, then keep it warm for 2-3 hours, then cool down to -5°C-5°C, more preferably 0°C, add water and filter 1. Obtain the crude product of the target product after washing with water; the aprotic polar solvent is dimethylformamide and / or dimethylacetamide, and the proportion of the compound of formula II and the aprotic polar solvent is 1g: (0.6~1.0)ml; The proportioning ratio of formula II compound and acetic anhydride is 1g: (0.6~1.0) ml; the mass ratio of formula II compound and 4-dimethylaminopyridine is 1: (0.08~0.12); The mass ratio of compound II is (0.3-0.5):1.
[0036] S2. The target product crude product described in step S1 is beaten and refined in a mix...
Embodiment 1
[0042] Add 50g of the compound of formula II, 50ml of acetic anhydride, 30ml of dimethylformamide and 4g of 4-dimethylaminopyridine to the reaction flask in sequence, stir and raise the temperature to 85-90°C, and then keep the reaction for 3 hours. Cool down to 0°C, add 20g of water dropwise, continue to stir at -5°C to 0°C for 0.5 hours, let stand for more than 2 hours, filter and wash with water to obtain the crude lorazepam intermediate. The crude product was added into a mixed solvent of 100 ml of acetone and 50 ml of water for beating and refining to obtain 52.6 g of a lorazepam intermediate, with an HPLC purity of 99.3% and a yield of 93.03%.
Embodiment 2
[0044] Add 50g of the compound of formula II, 30ml of acetic anhydride, 50ml of dimethylacetamide and 6g of 4-dimethylaminopyridine to the reaction flask in sequence, stir and raise the temperature to 85-90°C, and then keep the reaction for 2 hours. Cool down to -5°C, add 25g of water dropwise, continue to stir at -5°C to 0°C for 0.5 hours, let stand for more than 2 hours, filter, and wash with water to obtain the crude product of lorazepam intermediate. The crude product was added into a mixed solvent of 80 ml of acetone and 20 ml of water for beating and refining to obtain 52.9 g of a lorazepam intermediate, with an HPLC purity of 99.1% and a yield of 93.56%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com