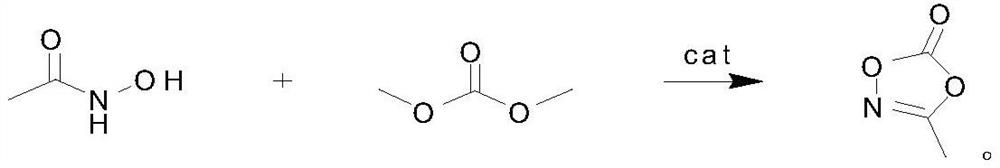
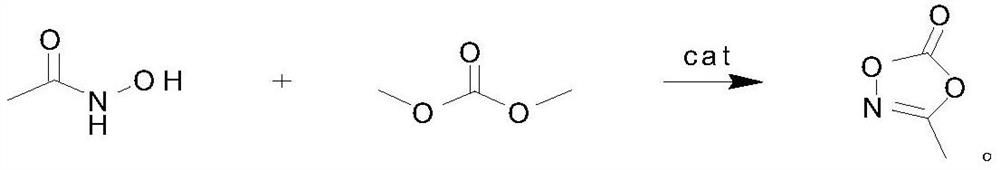
Preparation method of 3-methyl-1, 4, 2-dioxazole-5-one, product and application
A technology of dioxazole and methyl, applied in 3-methyl-1,4,2-dioxazol-5-one, 3-methyl-1,4,2-dioxazol-5-one In the field of preparation, it can solve the problems of less disclosure, achieve the effect of less by-products and reduce the purification steps in the later stage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 3-Methyl-1,4,2-dioxazol-5-one, its preparation method is carried out according to the following steps: first, 100 grams of acetohydroxamic acid, 240 grams of dimethyl carbonate and 2.4 grams of catalyst, wherein containing 1.59 grams of dialdehyde and 0.81 grams of calcium hydroxide were added to the reaction flask, then heated to 110°C, and the by-product methanol was fractionated while reacting. After 4 hours of reaction, the reaction solution was distilled under reduced pressure and collected at 89°C / 109 g of 3-methyl-1,4,2-dioxazol-5-one was obtained in a fraction of 10 mmHg, with a yield of 81% and a purity of 99.7%.
Embodiment 2
[0026] 3-Methyl-1,4,2-dioxazol-5-one, its preparation method is carried out according to the following steps: first, 200 grams of acetohydroxamic acid, 720 grams of dimethyl carbonate and 10 grams of catalyst, wherein containing ethyl 5.2 grams of dialdehyde and 6 grams of barium hydroxide were added to the reaction flask, then heated to 110°C, and the by-product methanol was fractionated while reacting. After 4 hours of reaction, the reaction solution was distilled under reduced pressure and collected at 89°C / 227 g of 3-methyl-1,4,2-dioxazol-5-one was obtained in a fraction of 10 mmHg, with a yield of 85% and a purity of 99.2%.
Embodiment 3
[0028]3-Methyl-1,4,2-dioxazol-5-one, its preparation method is carried out according to the following steps: first, 200 grams of acetohydroxamic acid, 700 grams of dimethyl carbonate and 6 grams of catalyst, wherein containing 4 grams of dialdehyde and 2 grams of calcium hydroxide were added to the reaction flask, then heated to 110°C, and the by-product methanol was fractionated while reacting. After 4 hours of reaction, the reaction solution was distilled under reduced pressure and collected at 89°C / 10 mmHg fraction, 219 g of 3-methyl-1,4,2-dioxazol-5-one was obtained, with a yield of 82% and a purity of 99.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com