Preparation method of oxazepam intermediate
A kind of intermediate, compound technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] Above-mentioned preparation method comprises the following steps:
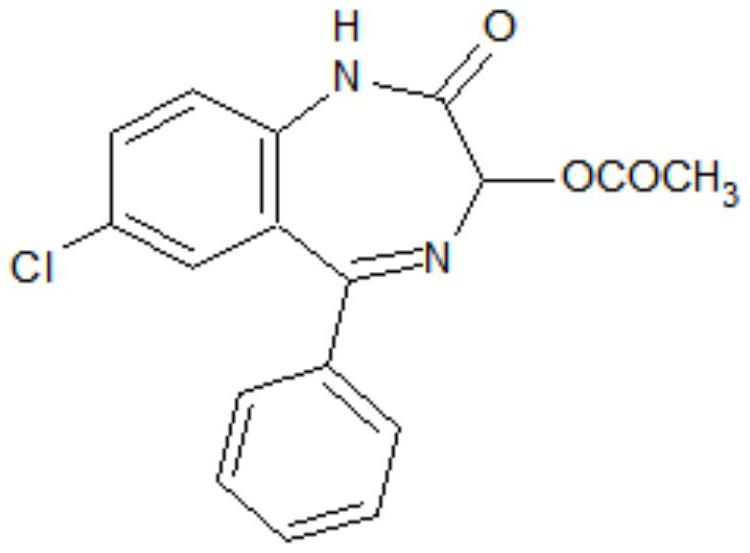
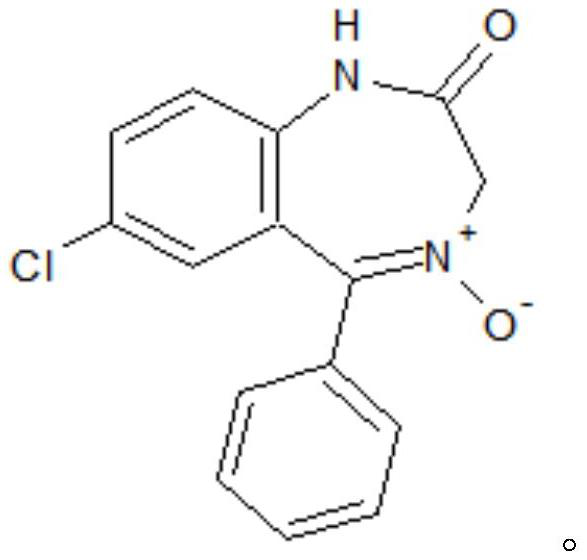
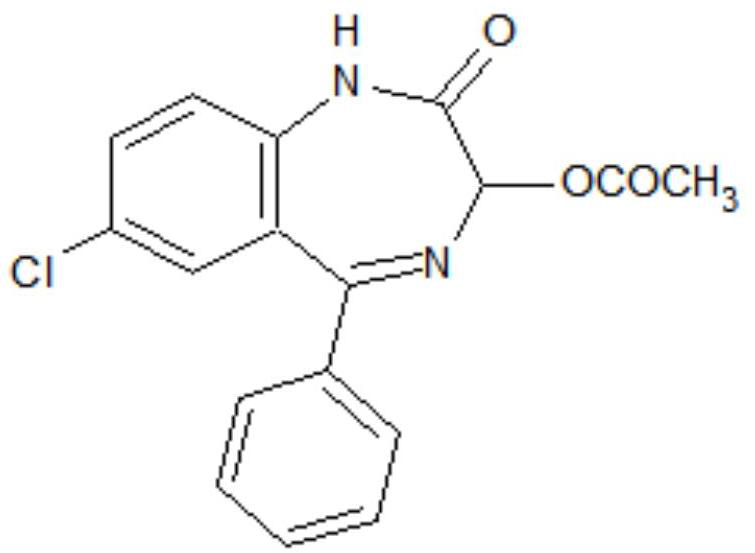
[0033] S1. To 7-chloro-2-oxo-5-phenyl-2,3-dihydro-1-H-1,4-benzodiazepine-4-oxide (hereinafter referred to as formula II compound) , acetic anhydride, and aprotic polar solvents, add anhydrous acetate, stir and heat up to 95°C to 100°C, then keep the temperature for 1.5-2.5h, then cool down to -5°C to 5°C after the reaction, more preferably 0°C , add water to precipitate, filter, and wash to obtain the crude product of the target product; the aprotic polar solvent is dimethylformamide and / or dimethylacetamide, and the proportioning of the formula II compound and the aprotic polar solvent is 1g: ( 0.6~1.0)ml; the ratio of formula II compound and acetic anhydride is 1g:(0.6~1.0)ml; the mass ratio of formula II compound and anhydrous acetate is 1:(0.1~0.2); The mass ratio of added water to the compound of formula II is (0.2-0.8):1, preferably (0.4-0.6):1.
[0034] S2. The target product crude product desc...
Embodiment 1
[0037] Add 30g 7-chloro-2-oxo-5-phenyl-2,3-dihydro-1-H-1,4-benzodiazepine-4-oxide, 24ml vinegar anhydride, 24ml of dimethylformamide and 3g of anhydrous sodium acetate, stirred and heated up to 95°C to 100°C, and then kept warm for 2 hours. Cool down to 0°C, add 12g of water dropwise, continue to stir at -5°C to 0°C for 0.5 hours, let stand for more than 2 hours, filter, and wash with water to obtain the crude oxazepam intermediate. The crude product was added into a mixed solvent of 90 ml of ethanol and 30 ml of water for beating and refining to obtain 31.8 g of an oxazepam intermediate with an HPLC purity of 99.4% and a yield of 92.4%.
Embodiment 2
[0039] Add 30g 7-chloro-2-oxo-5-phenyl-2,3-dihydro-1-H-1,4-benzodiazepine-4-oxide, 18ml vinegar anhydride, 20ml of dimethylformamide, 10ml of dimethylacetamide and 4.5g of anhydrous potassium acetate, stirred and heated to 95°C-100°C, and then kept at 95°C-100°C for 2.5 hours. Cool down to -5°C, add 18g of water dropwise, continue to stir at -5°C to 0°C for 0.5 hours, let stand for more than 2 hours, filter and wash with water to obtain the crude oxazepam intermediate. The crude product was added into a mixed solvent of 40 ml of ethanol and 20 ml of water for beating and refining to obtain 31.6 g of an oxazepam intermediate with an HPLC purity of 99.1% and a yield of 91.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com