Preparation method of high-purity LiNH2BH3 and NaNH2BH3
A high-purity and particle size technology, applied in the direction of borane/diborane hydride, etc., can solve the problems of difficult control of reaction temperature, low product purity, affecting hydrogen release performance, etc., to achieve product particle size controllability, process Simple, easy-to-use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Preparation of LiNH 2 BH 3
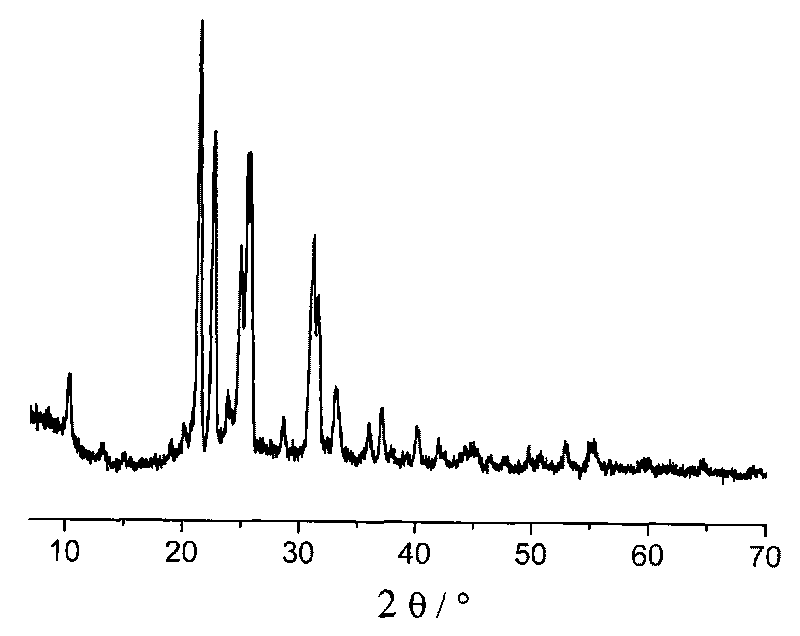
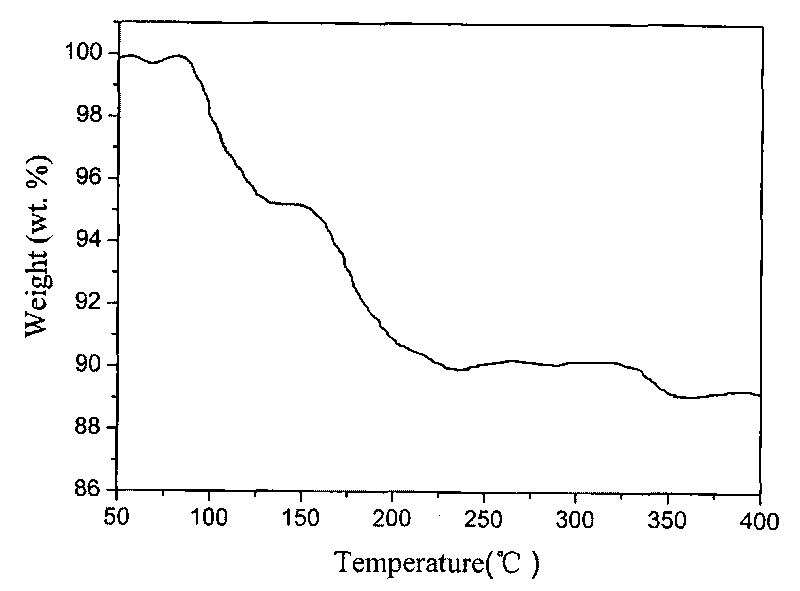
[0025] 1g BH at room temperature 25℃ 3 NH 3 Dissolve in 50ml of tetrahydrofuran in argon to dissolve completely. The above solution was slowly added to 0.2g LiH, after reacting for 1 hour, the solution was centrifuged, and the supernatant was decanted. After washing the product three times with 50 mL of tetrahydrofuran, the solid product after centrifugation was vacuum dried for 3 hours to obtain the final product ( figure 1 Is the XRD spectrum of the obtained product; figure 2 Is the thermogravimetric diagram of the product).
Embodiment 2
[0026] Example 2 Preparation of LiNH 2 BH 3
[0027] 1g BH at room temperature 25℃ 3 NH 3 Dissolve it in 50ml of ether in argon to dissolve completely. The above solution was slowly added to 0.2g LiH, after 30 minutes of reaction, the solution was centrifuged, and the supernatant was decanted. After washing the product three times with 50 mL of tetrahydrofuran, the solid product after centrifugation was vacuum dried for 3 hours to obtain the final product ( figure 1 Is the XRD spectrum of the obtained product; figure 2 Is the thermogravimetric diagram of the product).
Embodiment 3
[0028] Example 3 Preparation of NaNH 2 BH 3
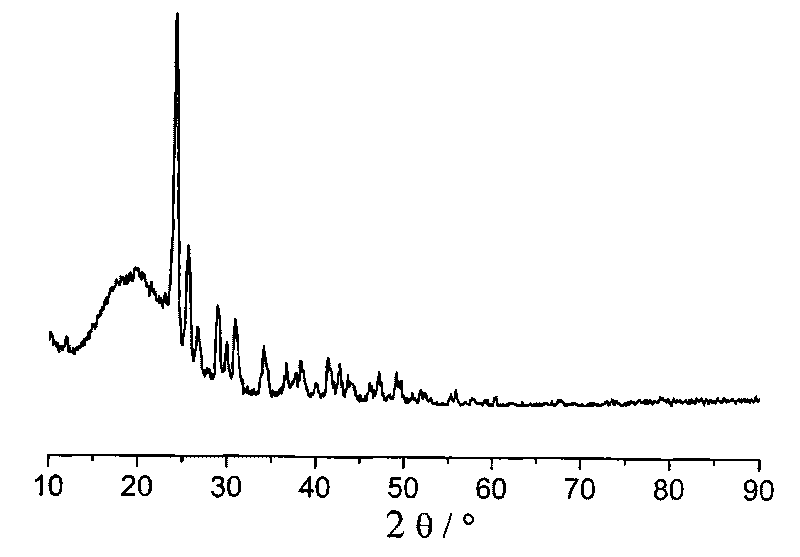
[0029] 1g BH at room temperature 25℃ 3 NH 3 Dissolve in 50ml of tetrahydrofuran in argon to dissolve completely. The above solution was slowly added to 0.5g NaH, and after reacting for 1 hour, the solution was centrifuged, and the supernatant was decanted. After washing the product three times with 50 mL of tetrahydrofuran, the solid product after centrifugation was vacuum dried for 3 hours to obtain the final product ( image 3 Is the XRD spectrum of the resulting product).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com