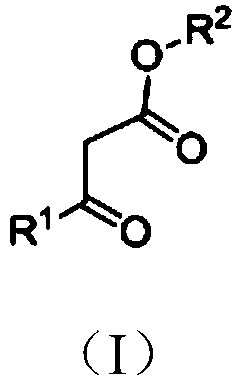
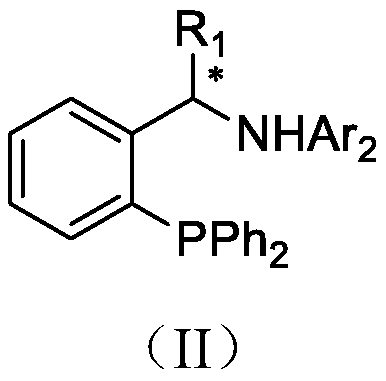
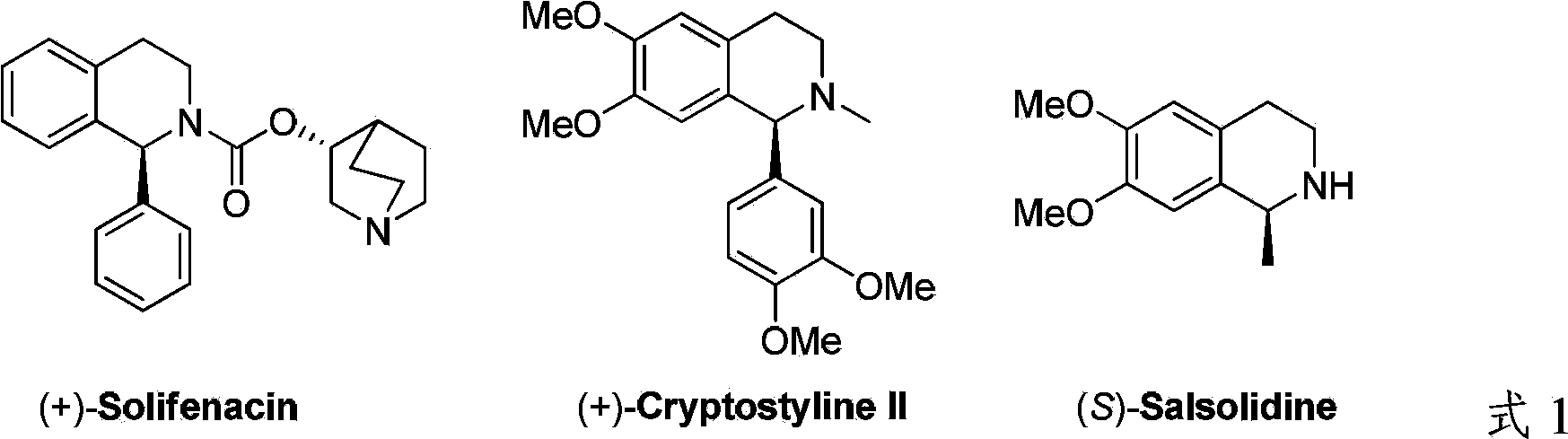
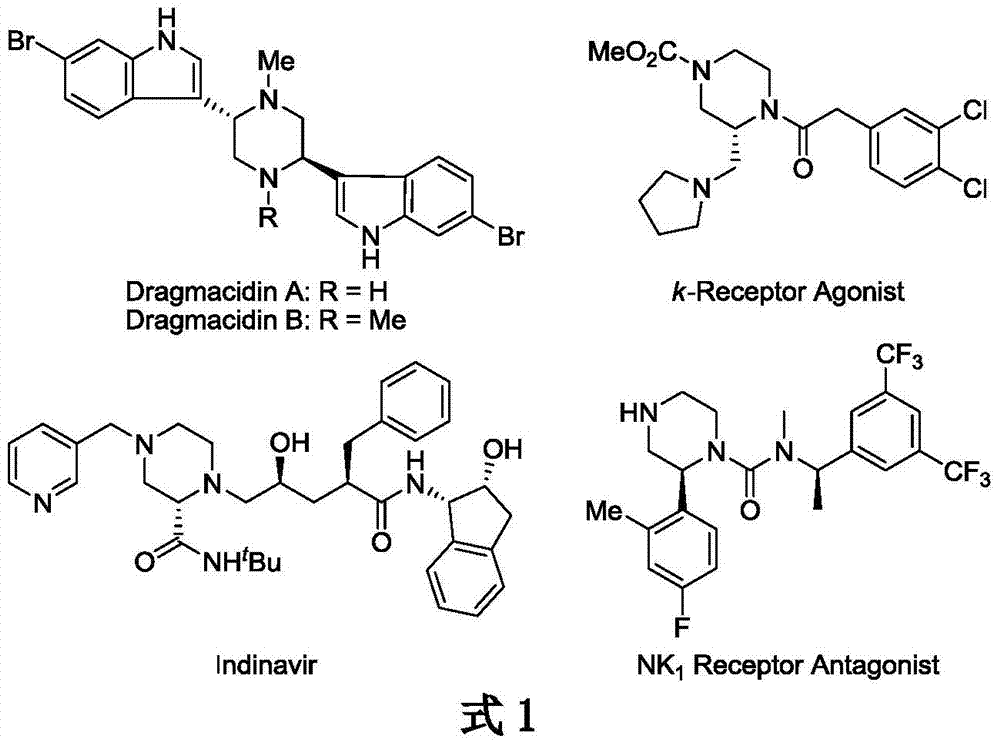
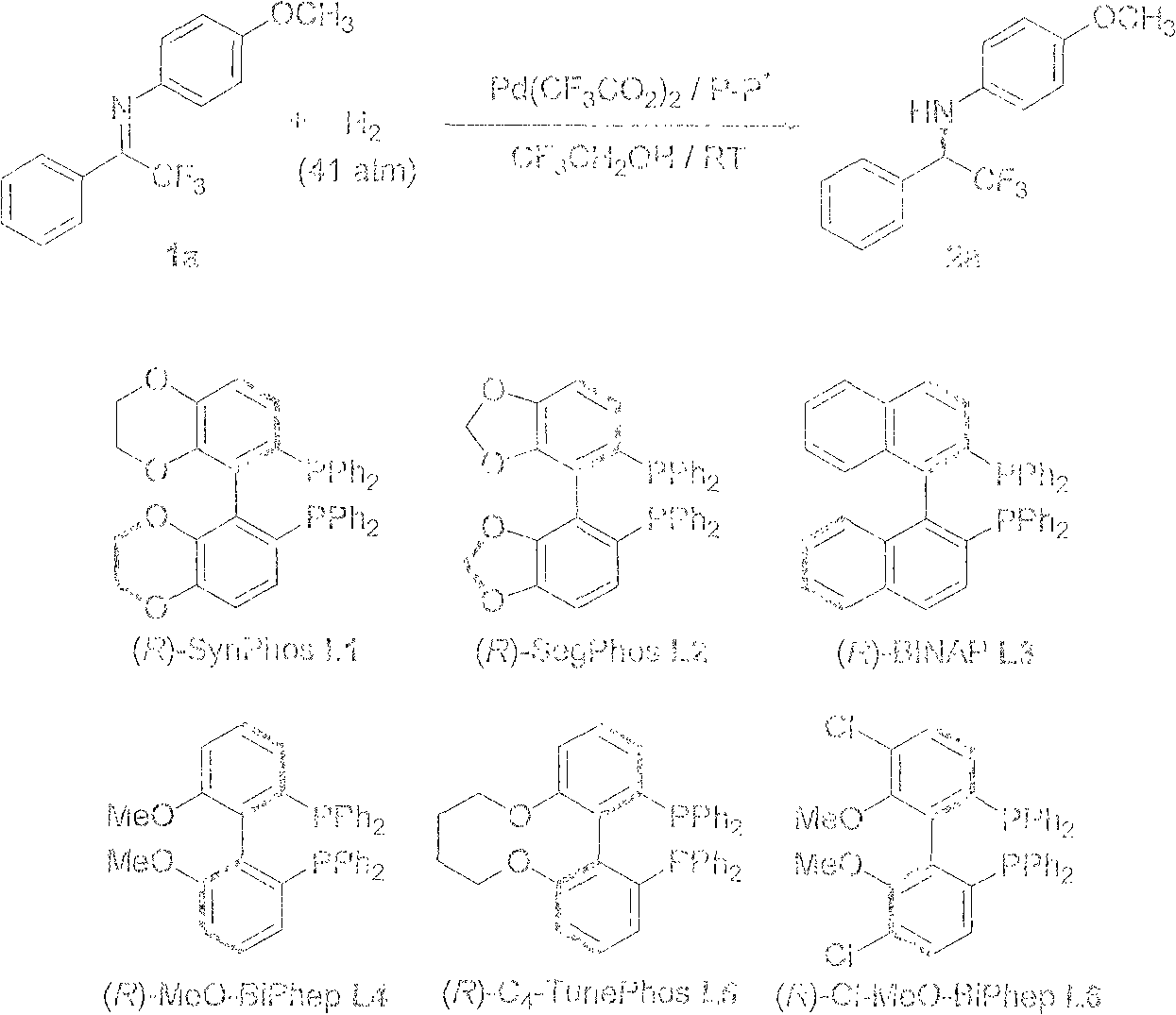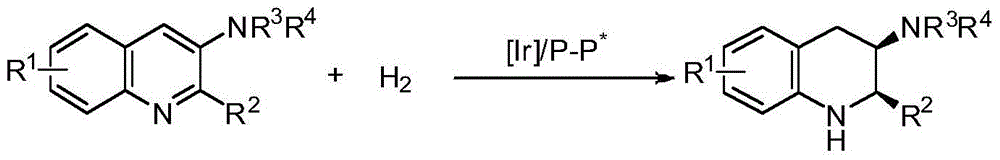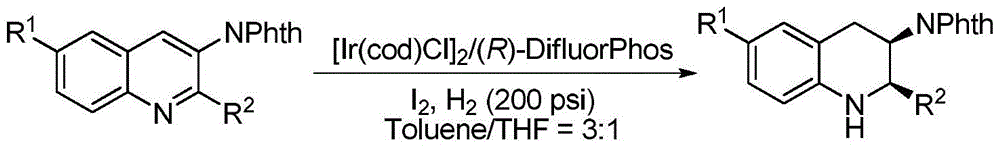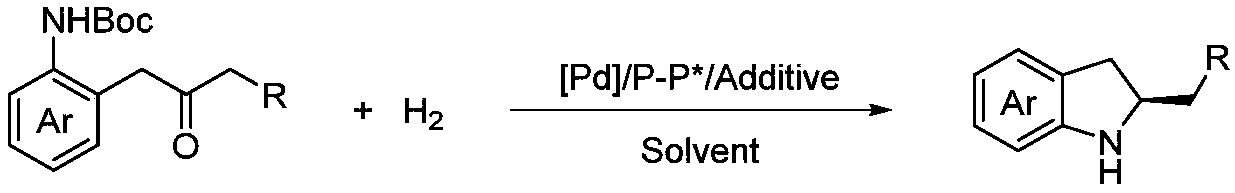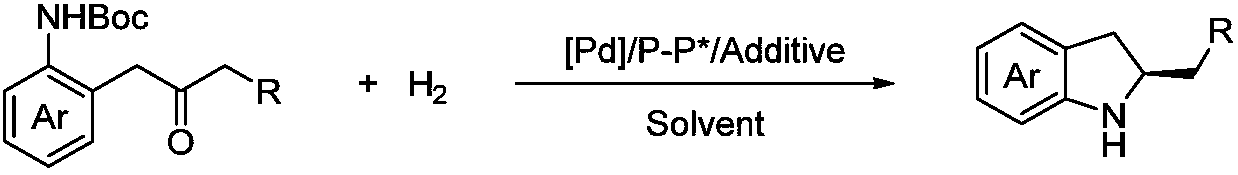Patents
Literature
50results about How to "The hydrogenation reaction conditions are mild" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
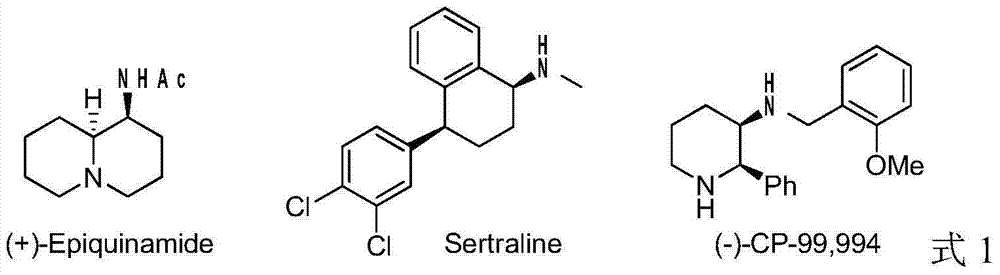
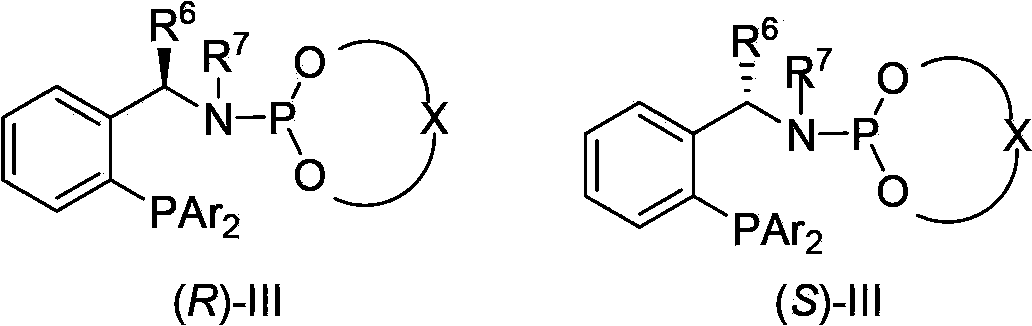
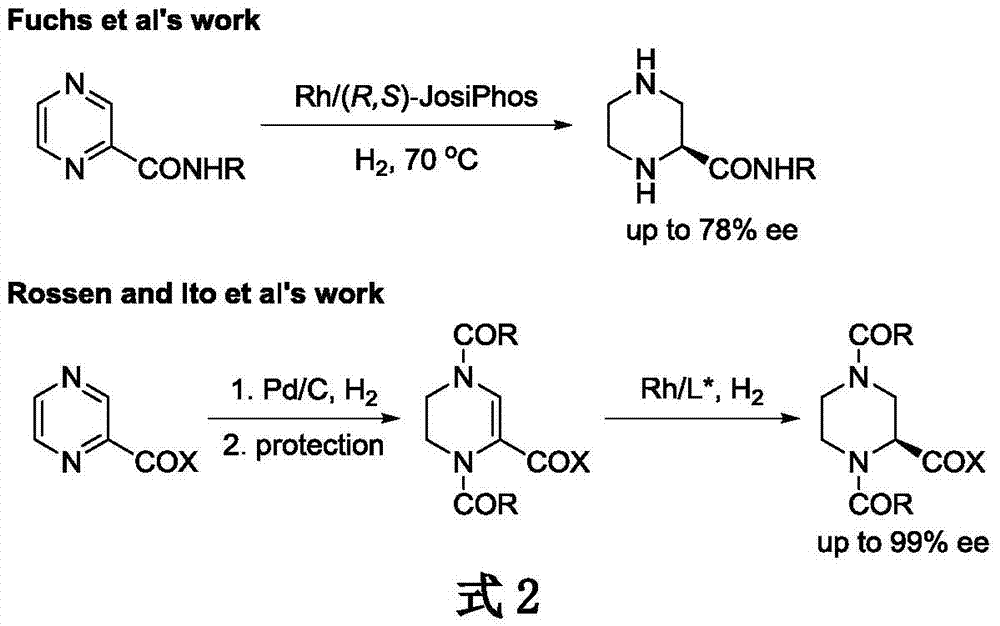
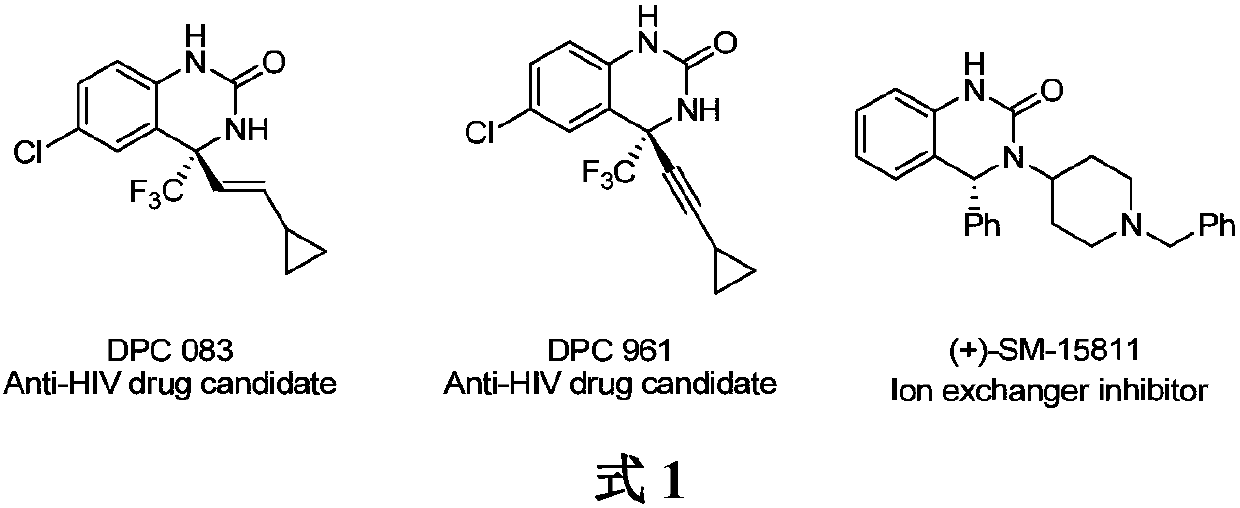
Method for synthesizing chiral cyclic amine through catalyzing asymmetric hydrogenation of quinolin-3-amine by iridium
ActiveCN104710406AEasy to separateHigh reactivityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsDiphosphinesIridium chloride
A catalysis system used by a method for synthesizing chiral cyclic amine through catalyzing asymmetric hydrogenation of quinolin-3-amine by iridium is a chiral diphosphine complex of iridium. A reaction is carried out under the following conditions: the temperature is 25-70DEG C; a solvent is a toluene / tetrahydrofuran mixed solvent (V / V=3:1); the pressure is 2-14atm; a ratio of a substrate to a catalyst is 25:1; and the catalyst is a (1,5-cyclooctadiene)iridium chloride dimer and chiral diphosphine ligand complex. A corresponding chiral cyclic amine derivative is prepared from quinolin-3-amine, and the enantiomeric excess value can reach 94%. The method has the advantages of simple and practical operation, easily available raw material, high enantioselectivity, good yield, and green, atom-economic and environmentally-friendly reaction.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for synthesizing chiraltetrahydro naphthalenederivate through asymmetric hydrogenation on isoquinoline by means of iridium catalyst
The invention discloses a method for synthesizing a chiraltetrahydro naphthalenederivate through asymmetric hydrogenation on isoquinoline by means of an iridium catalyst. A catalysis system applied in the method is a chiral bi-phosphine complex of metal iridium. The reaction is carried out under the conditions that the temperature is 25-60 DEG C; the volume ratio of tetrahydrofuran to dichloromethane in a solvent, namely a mixed solvent of tetrahydrofuran and dichloromethane is 1:1; the pressure is 13-50 Mpa; the ratio of a substrate to a catalyst is 50:1; the catalyst is a coordination compound of a (1,5-cyclooctadiene) iridium chloridedipolymer and a chiral bi-phosphine ligand. The corresponding chiral 1-position or 3-position substituted tetrahydro naphthalenederivate through hydrogenation on isoquinoline, and the enantiomeric excess of the derivate can reach 96%. The method is simple and practical in operation, raw materials are easy to obtain, the enantioselectivity is high, the yield is high, the reaction has atom economy, and the environment is friendly.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
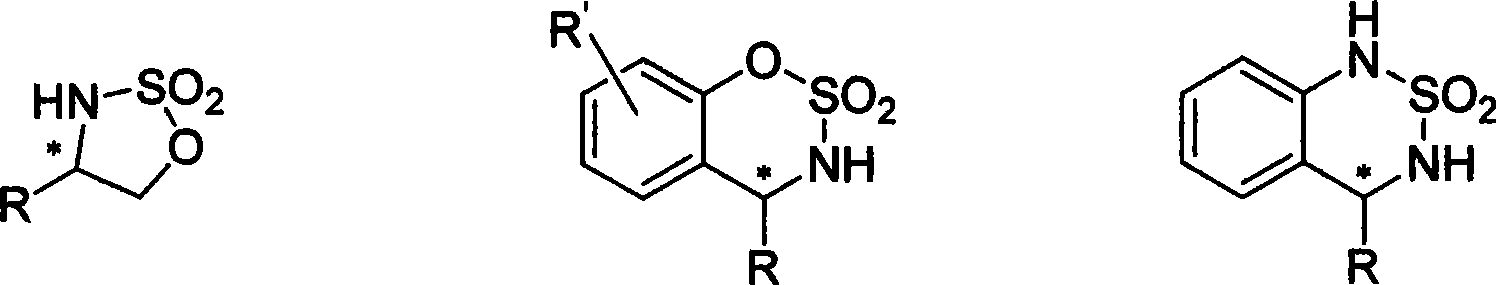
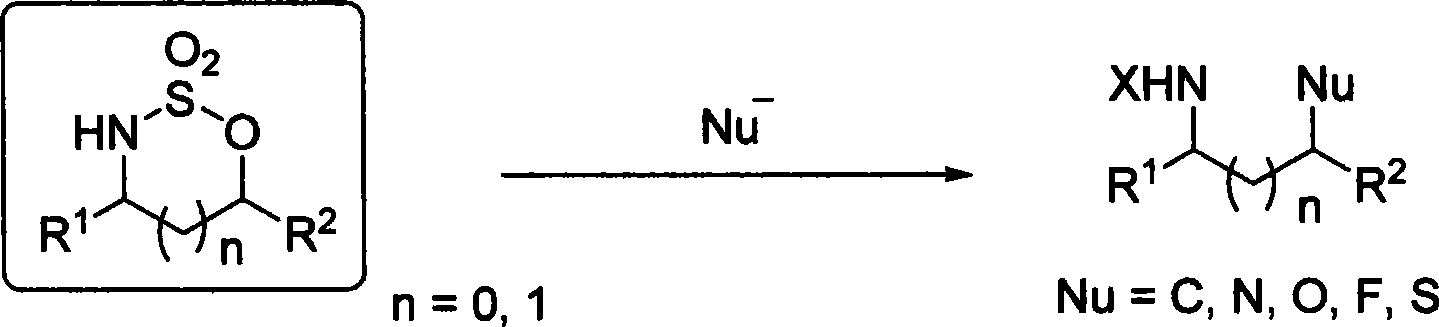
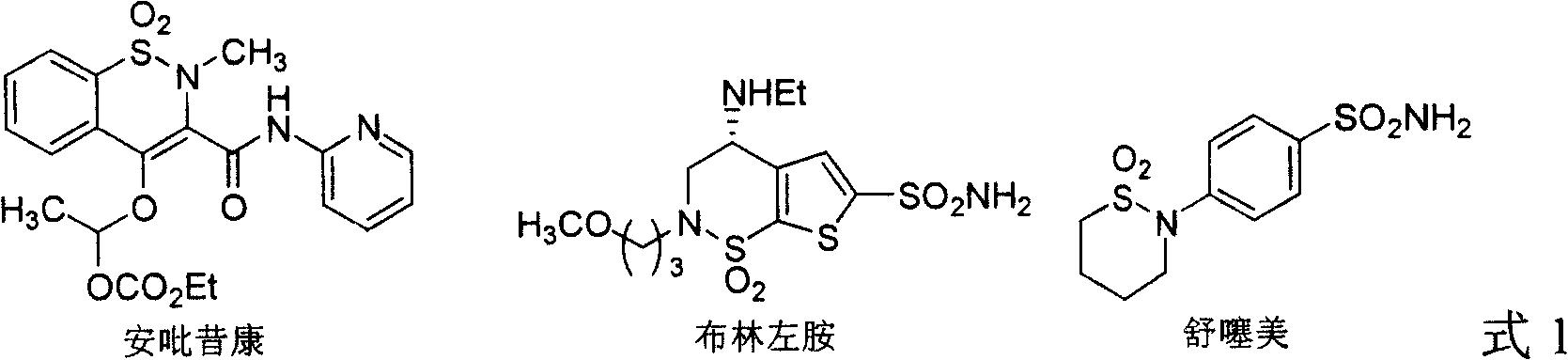
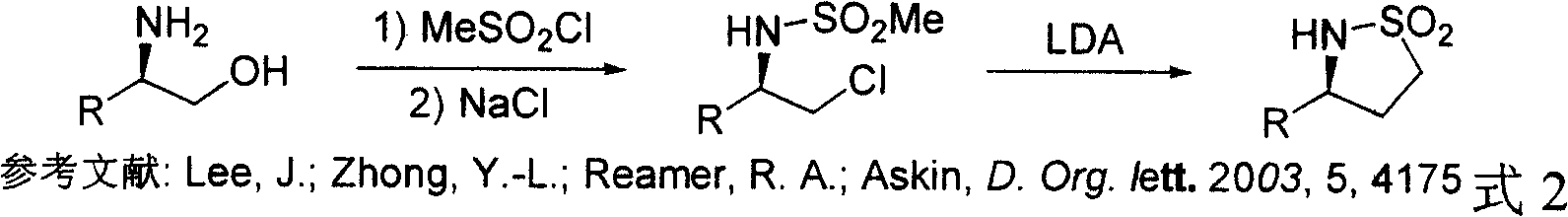
Method for synthesizing chiral sultam containing hetero atom unsymmetrical hydrogenation using Pd as catalyst
InactiveCN101423504AHigh reactivity and enantioselectivityRespond completelyOrganic-compounds/hydrides/coordination-complexes catalystsAsymmetric synthesesSolvent2,2,2-Trifluoroethanol
The invention provides a method for synthesizing chiral sultam containing hetero atoms by palladium-catalyzed asymmetric hydrogenation, wherein the catalyst is a chiral diphosphine ligand of palladium. The reaction is carried out at a temperature of between 25 and 60 DEG C and at a pressure of 40 atmosphere for 12 hours in solvent of 2, 2, 2-trifluoroethanol. Sulfonyl oxathiazinane heterocycle five-membered cyclic imide is hydrogenated to obtain corresponding chiral sultam containing oxathiazinane heterocycles with enantiomeric which excessively reaches 97 percent; and benzo N-sulfonyl oxathiazinane heterocycle six-membered cyclic imide is hydrogenated to obtain corresponding chiral sultam containing oxathiazinane heterocycles with enantiomeric which excessively reaches 99 percent; and benzo N-sulfonyl benzothiazinane heterocycle six-membered cyclic imide is hydrogenated to obtain corresponding chiral sultam containing benzothiazinane heterocycles with enantiomeric which excessively reaches 98 percent. The method has the advantages of simple and practical operation, high enantiomeric selection and good yield, and the reaction has green atomic economy and is friendly to environment.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
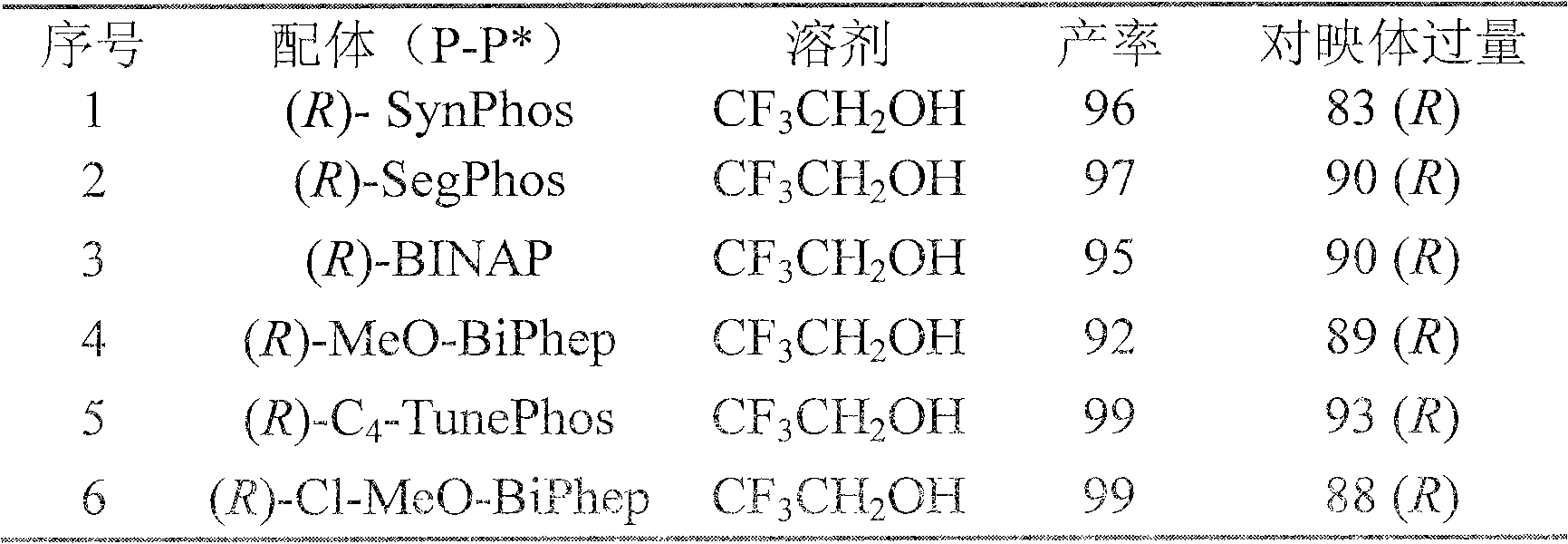
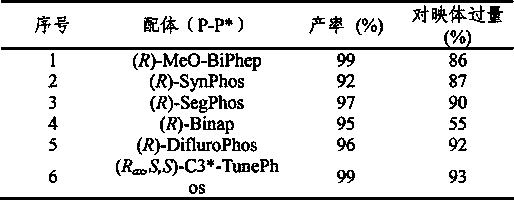
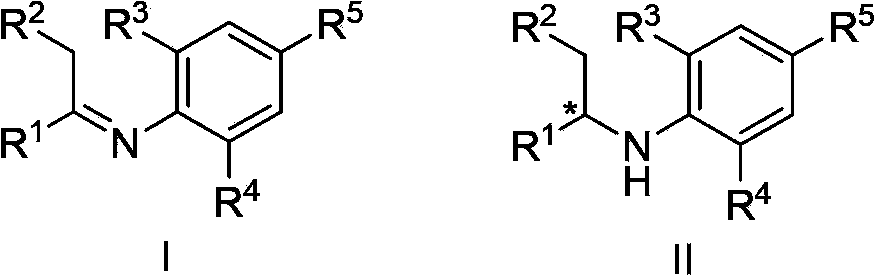
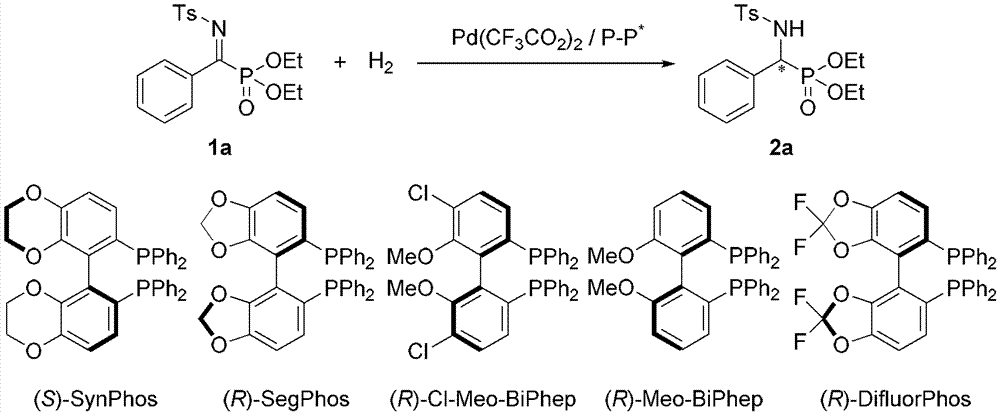
Asymmetric hydrogenation synthetic method of chiral aromatic amine compound with high steric hindrance
InactiveCN103570484AThe hydrogenation reaction conditions are mildSmooth responseOrganic compound preparationCarboxylic acid amides preparationIridiumPotassium iodine
The invention relates to an asymmetric hydrogenation synthetic method of a chiral aromatic amine compound of iridium-catalyzed imine with high steric hindrance. The catalyst adopted is a catalyst generated by in-situ reaction of a 1, 5-cyclooctadiene iridium chloride dimer and a chiral phosphine-phosphoramidite ligand. The reaction can be carried out under the following condition: the additives comprise iodine, potassium iodide and tetrabutyl ammonium iodide; the temperature is 0-200 DEG C; the solvent is dichloromethane, 1, 2-dichloroethane to the like; the pressure is 10-100 barometric pressures; the time is 12-48 hours; and the proportion of a primer and the catalyst can reach 100000 / 1. The method provided by the invention has the characteristics of mild reaction condition, high activity, high stereoselectivity, wide application range of the primer and the like.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
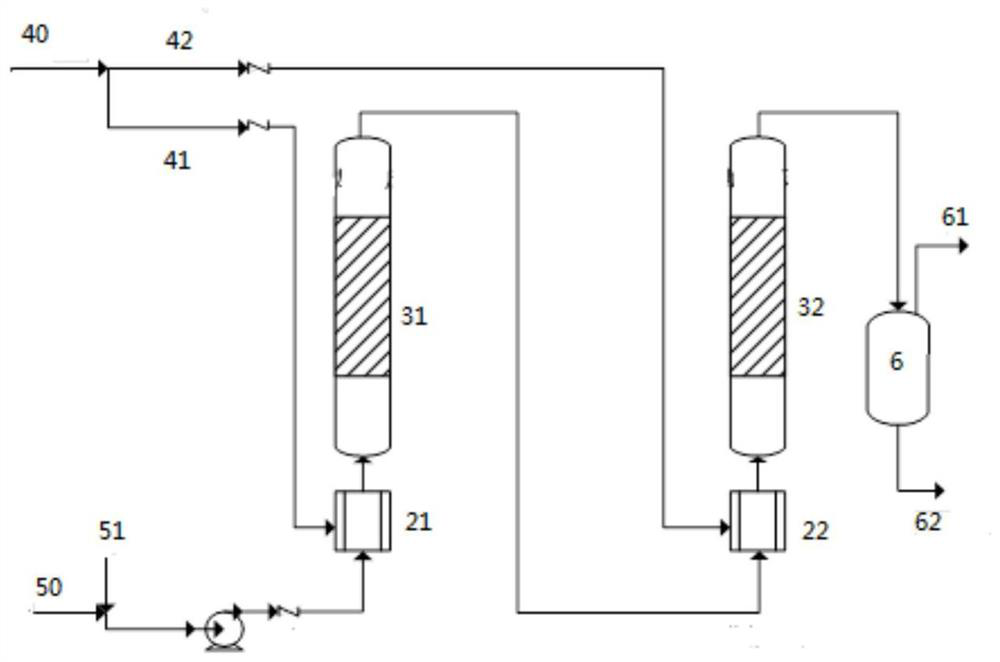
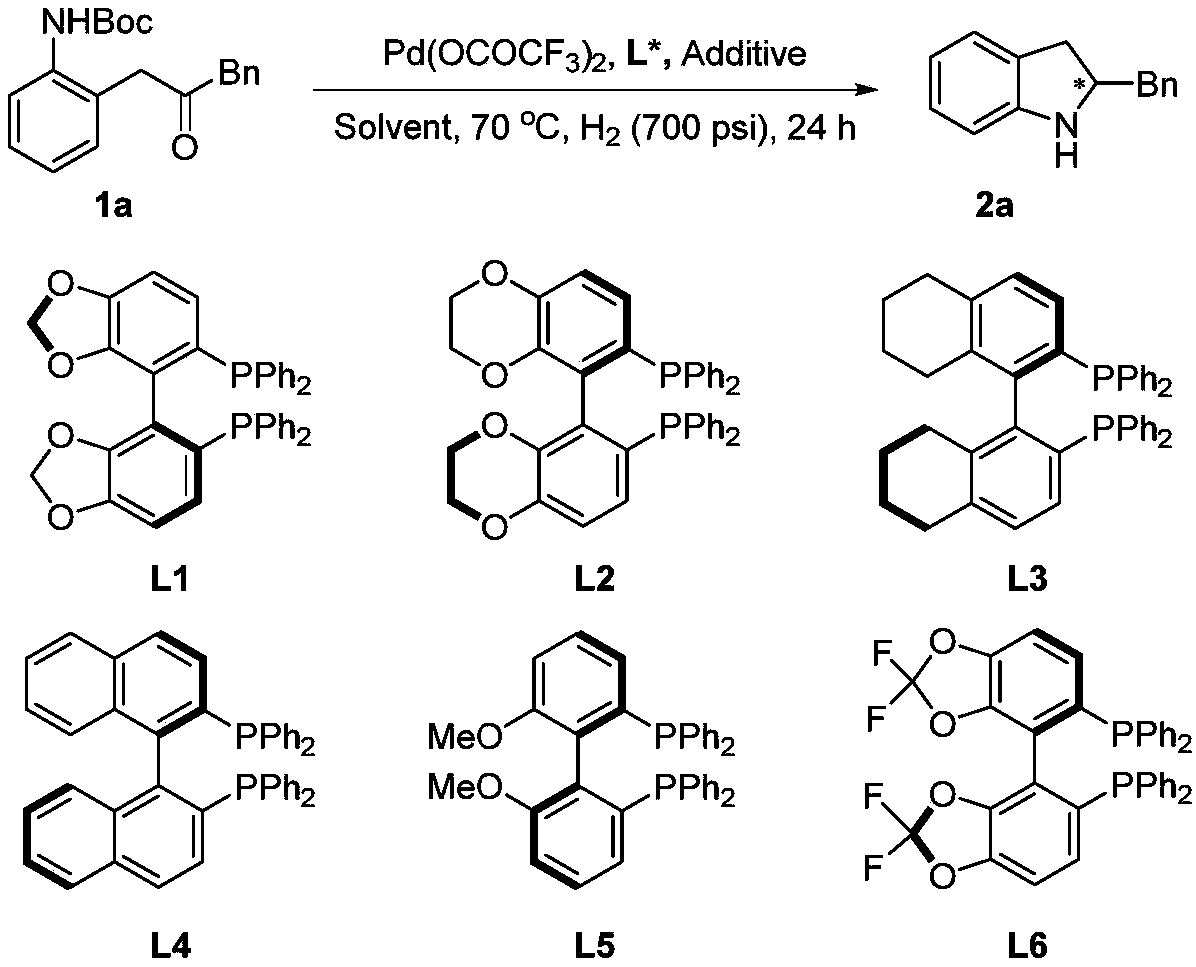
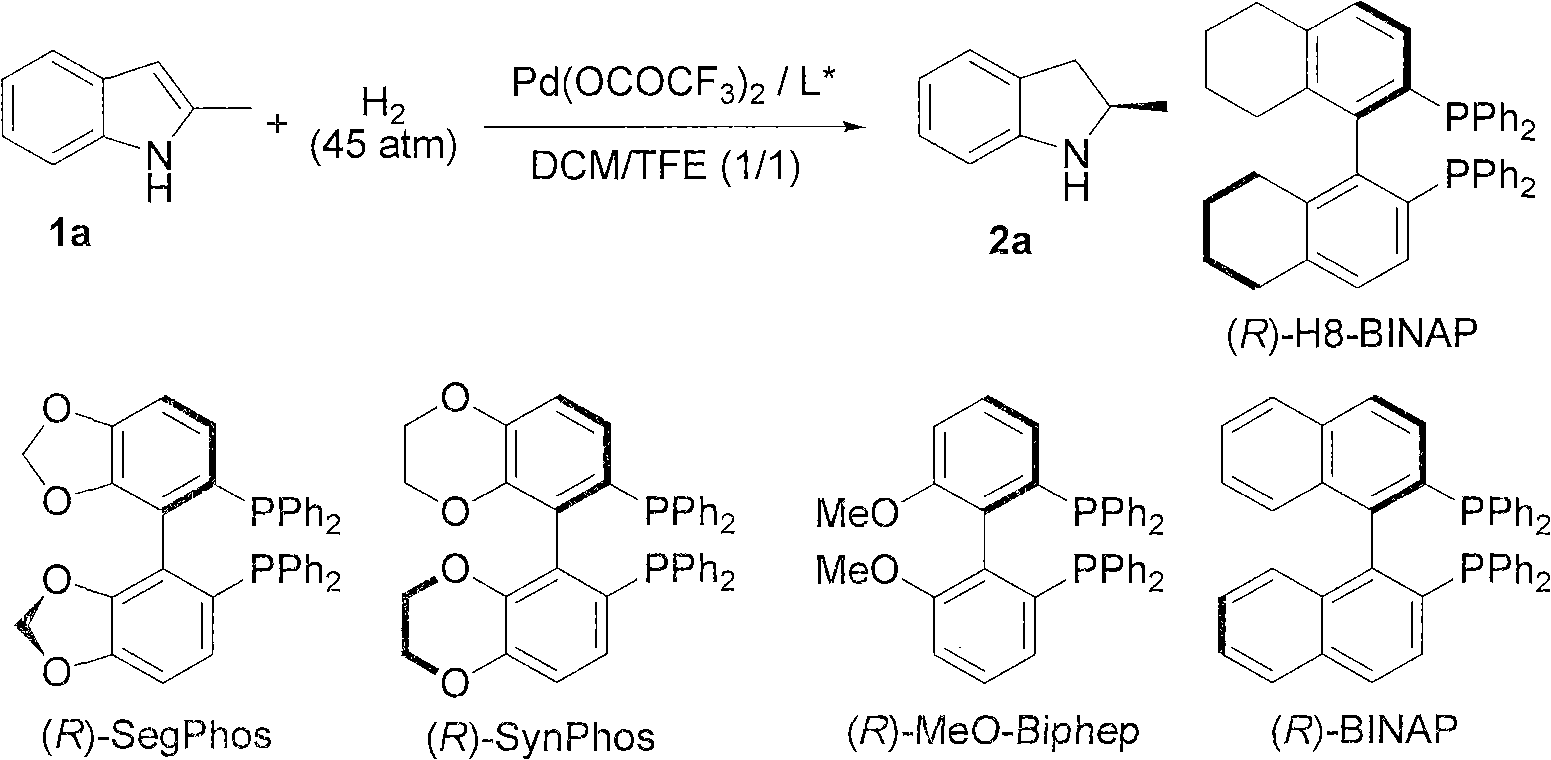
Method for synthesizing chiral indoline through palladium-catalyzed asymmetric hydrogenation
InactiveCN102336698AAsymmetric hydrogenationHigh reactivityOrganic-compounds/hydrides/coordination-complexes catalystsAsymmetric synthesesHydrogen pressureReaction temperature
The invention relates to a method for synthesizing chiral indoline through palladium-catalyzed asymmetric hydrogenation, which is characterized in that Bronsted acid is used as the activator and the used catalysis system is a palladium chiral diphosphine complex. The reaction can be carried out under the following conditions: the reaction temperature is 20-50 DEG C; the solvent used in the reaction is a mixed solvent of dichloromethane and 2,2,2-trifluoroethanol (the DCM / TFE volume ratio is 1:1); the hydrogen pressure is 13-45 atmospheres; the substrate-catalyst ratio is 50 / 1; the used metal precursor is palladium trifluoroacetate (Pd(OCOCF3)2); the used chiral ligand is a chiral diphosphine ligand; the used activator is L-camphorsulfonic acid (L-CSA); and unprotected simple 2-substituted indole and 2,3-disubstituted indole can be hydrogenated to obtain the corresponding chiral indoline product, and the enantiomeric excess can be up to 96%. The method has the advantages of convenient operation process, high practicality, easy acquisition of raw materials, good enantioselectivity and high yield.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Catalytic asymmetric hydrogenation synthesis method for chiral gamma-sultam
InactiveCN101260085AHigh reactivityHigh enantioselectivityOrganic chemistrySynthesis methodsDiphosphines
The invention discloses a method using palladium to catalyze asymmetrical hydrogen to compound into chiral gamma-sultam, wherein, a catalytic system used by the invention is a chiral diphosphine complex of palladium. The reaction can be made within the following conditions: temperature: 25 to 75 DEG C; solvent: 2, 2, 2-trifluoroethanol; pressure: 35 to 40 atmospheric pressures; time: 10 to 12 hours. The proportion of substrate and catalyst is 50:1, a metal precursor of the catalyst is palladium trifluoroacetate; a chiral ligand is a chiral diphosphine ligand; the preparation method for the catalyst is that: the metal precursor of the palladium and the chiral diphosphine ligand are stirred in acetone at room temperature, and then are made a vacuum condensation to obtain the catalyst. To hydrogenise cyclic sulfimide can obtain corresponding chiral 3-substituted gamma-sultam, and enantiomeric excess thereof can reach 93 percent. The method has simple and practical operation, easy-obtaining raw materials, high enantioselectivity and good yield; the reaction has green atomic economy and is environment-friendly.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for synthesizing chiral phosphoramidate by carrying out asymmetric hydrogenation on palladium-catalyzed imine phosphate
InactiveCN106866730AHigh reactivityHigh enantioselectivityGroup 5/15 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsPhosphateAsymmetric hydrogenation
The invention discloses a palladium-catalyzed method for asymmetric hydrogenation of imine phosphonate to synthesize chiral amino phosphonate. The catalyst system used is palladium chiral bisphosphorus complex. Asymmetric hydrogenation of a series of imine phosphonates can give the corresponding chiral amino phosphonates with an enantiomeric excess of 99%. The invention is simple and practical to operate, the catalyst is commercially available, and the reaction conditions are mild. In addition, the synthesis of chiral aminophosphonates by asymmetric hydrogenation has high enantioselectivity and good yield, and the reaction has green atom economy and is environmentally friendly.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
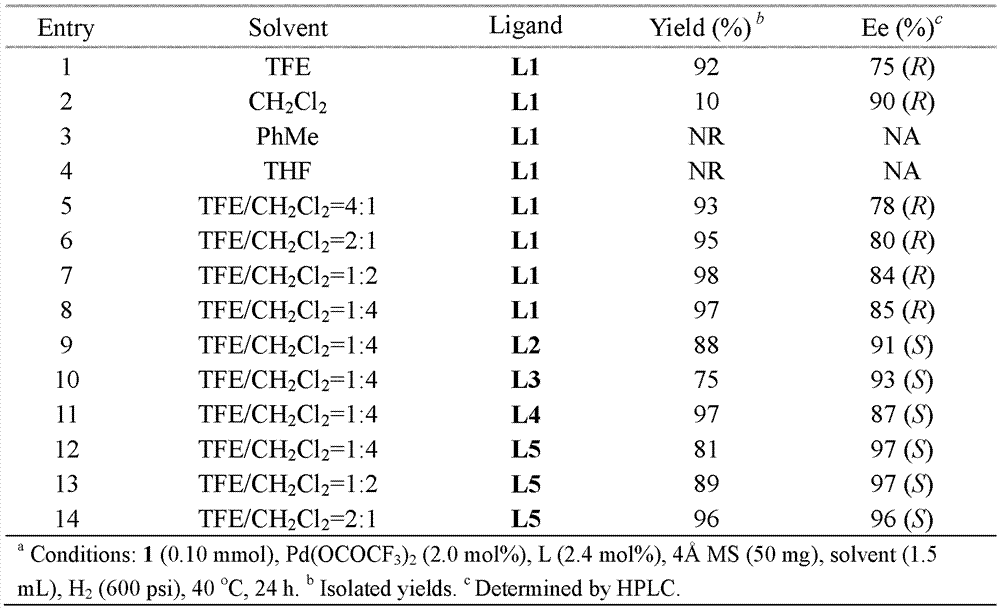
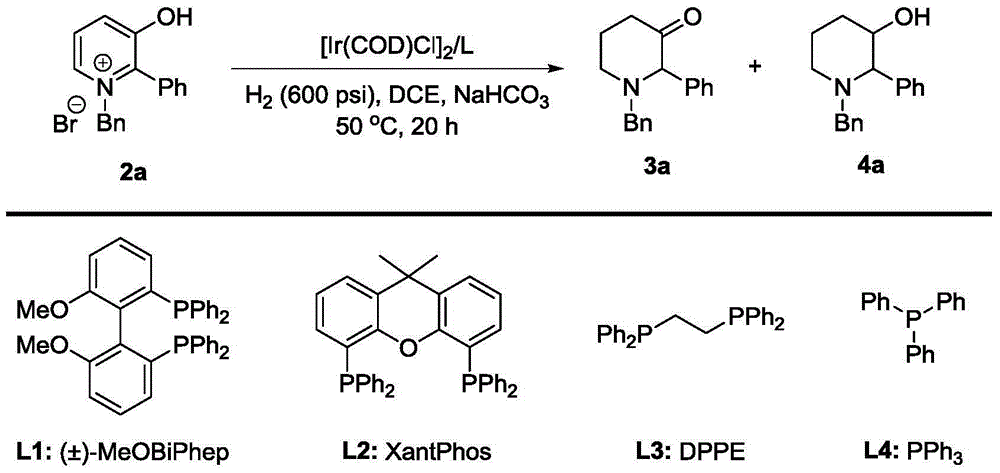
Method for synthesis of 3-piperidone derivatives through iridium catalytic hydrogenation
A method for synthesis of 3-piperidone derivatives through iridium catalytic hydrogenation is provided, wherein a used catalyst is a triphenylphosphine complex of iridium. A reaction can be carried out under the following conditions: the temperature is 40-60 DEG C; the solvent is 1,2-dichloroethane; the pressure is 20-50 atmospheric pressures; the ratio of a substrate to a catalyst is 100 to 1; and the catalyst is a complex of chloro(1,5-cyclooctadiene)iridium(I) dimer and triphenylphosphine. Hydrogenation of 3-hydroxypyridine benzyl bromide salt can obtain the 3-piperidone derivatives with excellent chemoselectivity, the highest yield can reach 97%, and the chemical selectivity of ketones and alcohols is greater than 20:1. The method has the advantages of simple and convenient operation, easily obtained raw materials, high chemoselectivity, and good yield, and provides an atom economic and environment-friendly route for synthesis of a series of 3-piperidone derivatives.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for synthesizing chiral dihydro-5H-pyrrolo[2,1-c][1,4]-benzodiazepino-5-one
InactiveCN103288829AHigh reactivityHigh enantioselectivityAsymmetric synthesesMorpholineDiphosphines
The invention relates to a method for synthesizing chiral dihydro-5H-pyrrolo[2,1-c][1,4]-benzodiazepino-5-one by iridium-catalyzed asymmetric hydrogenation. The reaction conditions are as follows: the temperature is 20-50 DEG C; the solvent is a dichloromethane-toluene mixed solvent (V / V=1:2); the pressure is 13-50 atmospheric pressures; the ratio of the substrate to the catalyst is 50 / 1; the catalyst is a complex of (1,5-cyclooctadiene)iridium chloride dimer and diphosphine ligand; and the additive is morpholine trifluoroacetate or piperidine hydrochloride. Seven-element cyclic pyrrolo[2,1-c][1,4]-benzodiazepino-5-one is hydrogenated to obtain the corresponding chiral dihydro product, and the enantiomeric excess can reach 96%. The invention is simple and practical to operate, and has the advantages of accessible raw materials, favorable selectivity for antipode, high yield, atomic economical efficiency for reaction, and environment friendliness.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for synthesizing chiral dihydro-6H-indolo[2,1-c][1,4]-benzodiazepine
InactiveCN103288828AHigh reactivityHigh enantioselectivityOrganic chemistryBenzodiazepineDiphosphines
The invention relates to a method for synthesizing chiral dihydro-6H-indolo[2,1-c][1,4]-benzodiazepine by iridium-catalyzed asymmetric hydrogenation. The reaction conditions are as follows: the temperature is 0-50 DEG C; the solvent is benzene or toluene; the pressure is 13-60 atmospheric pressures; the ratio of the substrate to the catalyst is 50 / 1; and the catalyst is a complex of (1,6-cyclooctadiene)iridium chloride dimer and diphosphine ligand. Seven-element cyclic indolo[2,1-c][1,4]-benzodiazepine is hydrogenated to obtain the corresponding chiral dihydro product, and the enantiomeric excess can reach 89%. The invention is simple and practical to operate, and has the advantages of accessible raw materials, favorable selectivity for antipode, high yield, atomic economical efficiency for reaction, and environment friendliness.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
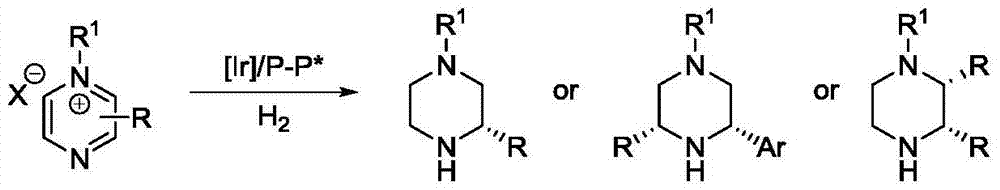
Iridium catalytic method for asymmetric hydrogenation to synthesize piperazine derivatives
InactiveCN106995413AHigh reactivityHigh enantioselectivityOrganic chemistry methodsCatalytic methodPyrazine
In an iridium catalytic method for asymmetric hydrogenation to synthesize piperazine derivatives, an iridium chiral diphosphine complex is used as a catalyst. The reaction is carried out in the following condition that the temperature is in a range of -20 to 70 DEG C, the solvent is toluene, tetrahydrofuran and ethyl acetate, the pressure is 10-80 times of atmospheric pressure, the ratio of a substrate to the catalyst is 50 / 1, and the catalyst is a complex of (1,5-cyclo-octadiene) iridium chloride dimer and chiral diphosphine. 3-substitued, 3,5-disubstitued, and 2,3-disubstitued pyrazine salt can all be well hydrogenated to obtain corresponding chiral piperazine derivatives, with the highest enantiomeric excess reaching 96%. The method is simple to operate, available in raw materials, high in enantioselectivity, and good in yield, and an atom-economic eco-friendly route is provided for synthesizing 3-substitued, 3,5-disubstitued, and 2,3-disubstitued piperazine derivatives.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Hydrogenation catalyst and application thereof as well as preparation method of cyclohexane-1, 2-diformylic acid dibasic ester
ActiveCN107774257AThe hydrogenation reaction conditions are mildLow reaction temperatureOrganic compound preparationCarboxylic acid esters preparationActivated carbonPtru catalyst
The invention discloses a hydrogenation catalyst and application thereof. The hydrogenation catalyst is prepared from a carrier as well as main active element(s) and additive elements which are loadedon the carrier, wherein the main active element(s) is / are Rh or / and Ru, the additive elements are selected from Sm, Eu and Yb, and the carrier is selected from activated carbon, Al2O3 and SiO2. The invention also discloses a method for preparing cyclohexane-1, 2-diformylic acid dibasic ester by using the hydrogenation catalyst. The hydrogenation catalyst provided by the invention has higher hydrogenation catalytic activity, also shows higher catalytic activity even at the lower reaction temperature when being used as the catalyst for preparing the cyclohexane-1, 2-diformylic acid dibasic ester by carrying out hydrogenation on diisonoyl phthalate dibasic ester, and has higher raw material conversion rate and product selectivity.
Owner:HUNAN CHANGLING PETROCHEM SCI & TECH DEV CO LTD
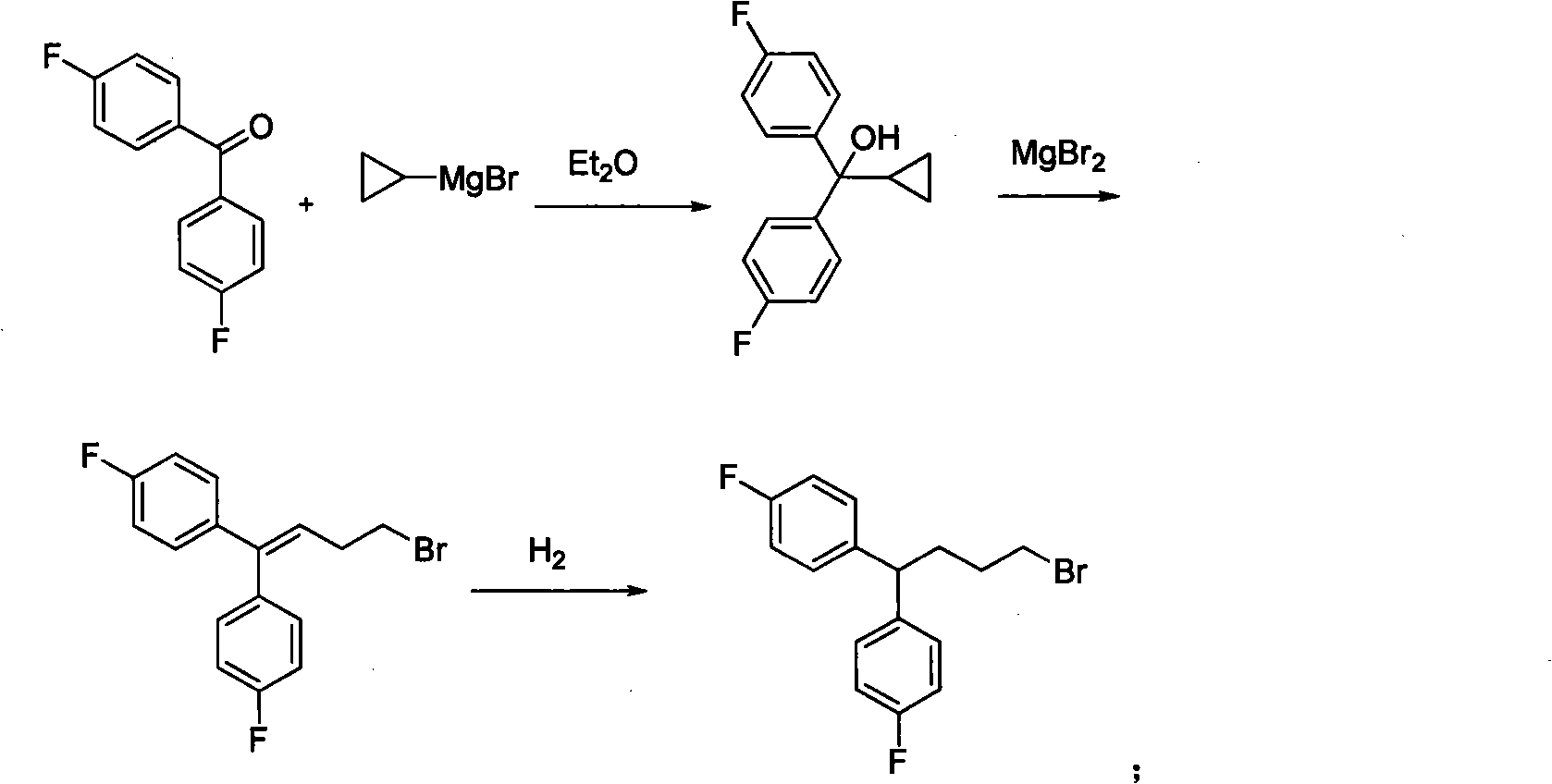
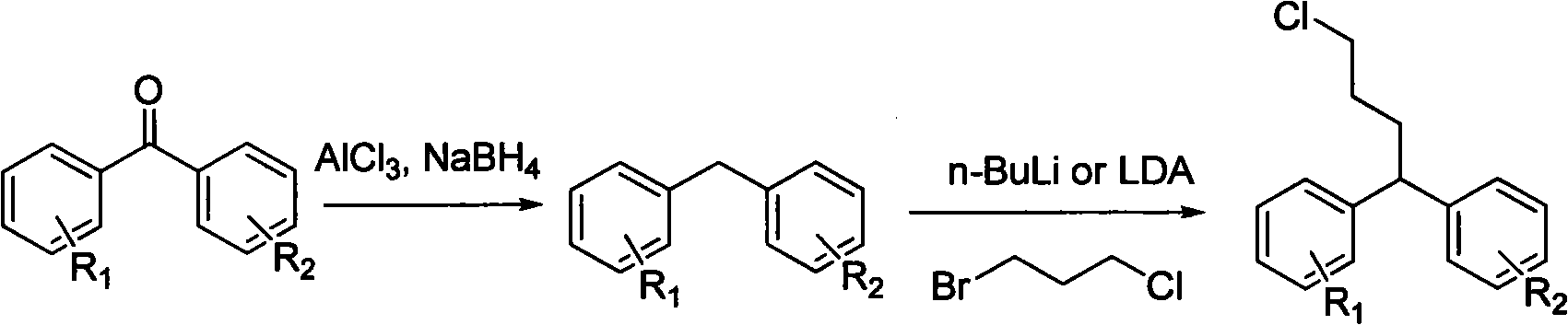
Diphenylalkyl halide or diphenyl carboxylic acid and synthesis method thereof
ActiveCN101948368AProne to formatting reactionsReaction raw materials are cheapOrganic compound preparationCarboxylic preparation by oxidationGrignard reagentSynthesis methods
The invention relates to diphenylalkyl halide or diphenyl carboxylic acid and a simple and convenient synthesis method thereof. The method comprises the following steps of: after a bromobenzene derivative and magnesium form a Grignard reagent, reacting the Grignard reagent and lactone, and opening loop to obtain 1,1-diphenyl-diol; reacting the 1,1-diphenyl-diol under the condition of a mixed solvent of an organic solvent and an acid to form diphenylenol; reacting the diphenylenol, a halogen or a halide and triphenylphosphine in the organic solvent to form diphenylalkenyl halogen; and reactingthe diphenylalkenyl halogen and hydrogen to form diphenylalkyl halide, or reacting the diphenylenol and hydrogen to form diphenyl alcohol; and reacting the diphenyl alcohol and CrO3 under the action of the organic solvent and the acid to form diphenyl carboxylic acid. The reaction raw materials are cheap; the reaction conditions are mild; and substitutional diphenylalkyl halide or diphenyl carboxylic acid compounds with different benzene rings can be synthesized.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Method for synthesizing chiral dihydro-5H-pyrrolo[2,1-c][1,4]-benzodiazepine
InactiveCN103288830AHigh reactivityHigh enantioselectivityAsymmetric synthesesBenzodiazepineDiphosphines
The invention relates to a method for synthesizing chiral dihydro-5H-pyrrolo[2,1-c][1,4]-benzodiazepine by iridium-catalyzed asymmetric hydrogenation. The reaction conditions are as follows: the temperature is 20-50 DEG C; the solvent is benzene or toluene; the pressure is 13-50 atmospheric pressures; the ratio of the substrate to the catalyst is 50 / 1; and the catalyst is a complex of (1,5-cyclooctadiene)iridium chloride dimer and diphosphine ligand. Seven-element cyclic pyrrolo[2,1-c][1,4]-benzodiazepine is hydrogenated to obtain the corresponding chiral dihydro product, and the enantiomeric excess can reach 96%. The invention is simple and practical to operate, and has the advantages of accessible raw materials, favorable selectivity for antipode, high yield, atomic economical efficiency for reaction, and environment friendliness.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Preparation method of 2-piperidinecarboxylic acid, 3-piperidinecarboxylic acid and 4-piperidinecarboxylic acid
InactiveCN102174011AThe reaction route is simpleLess side effectsOrganic chemistryHydrogenPipecolic acid
The invention discloses a preparation method of 2-piperidinecarboxylic acid, 3-piperidinecarboxylic acid and 4-piperidinecarboxylic acid. The method is as follows: 2-pyridinecarboxylic acid, 3-pyridinecarboxylic acid and 4-pyridinecarboxylic acid are used as the raw material respectively, hydrogen is used to reduce the raw material to the corresponding piperidinecarboxylic acid in the presence of palladium-carbon catalyst. The method has the advantages of simple route, less side reactions, low hydrogenation pressure and hydrogenation temperature, short hydrogenation time and simple treatment of solid wastes, and is easy to realize industrialization.
Owner:CHANGZHOU DAOU CHEM IND
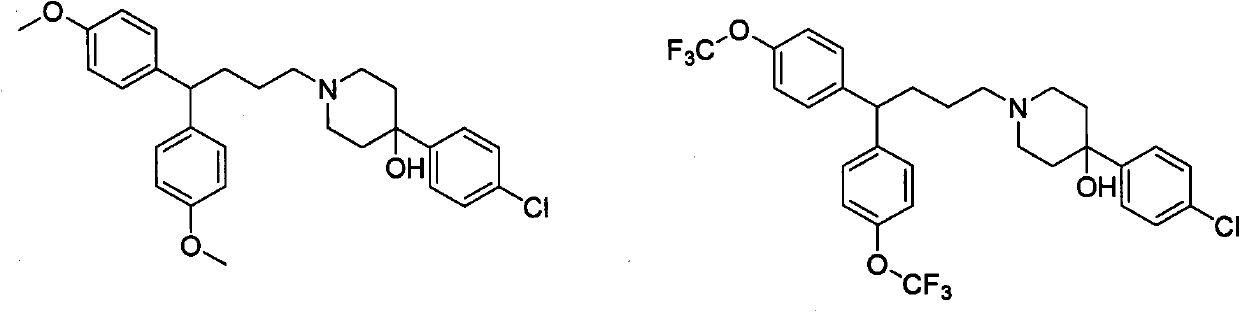
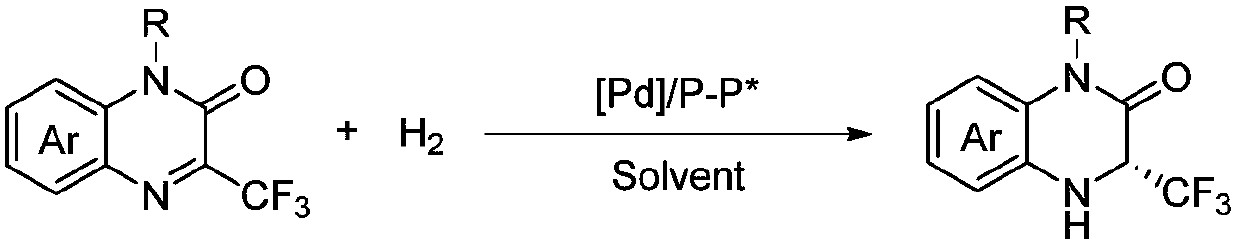
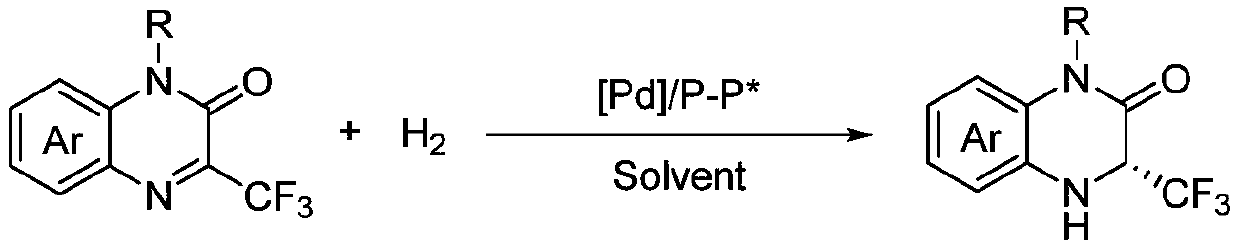
Method for synthesizing chiral 3-trifluoromethyl-3, 4-dihydroquinoxalinone by palladium-catalyzed asymmetric hydrogenation
The invention relates to a method for synthesizing chiral 3-trifluoromethyl-3, 4-dihydroquinoxalinone by palladium-catalyzed asymmetric hydrogenation. A catalytic system is a chiral diphosphorus complex of palladium, and the reaction conditions are as follows: the temperature is 0-80 DEG C, the solvent is 2, 2, 2-trifluoroethanol or hexafluoroisopropanol, the pressure ranges from 100 psi to 1000 psi, the ratio of the substrate to the catalyst is 33 / 1, the used metal precursor is palladium trifluoroacetate, and the used chiral ligand is a chiral diphosphorus ligand. The preparation method of the catalyst comprises the following steps: stirring a metal precursor of palladium and a chiral diphosphine ligand in acetone at room temperature, and then carrying out vacuum concentration to obtain the catalyst. Corresponding chiral dihydroquinoxalinone containing trifluoromethyl can be obtained by hydrogenating quinoxalinone containing trifluoromethyl, and the enantiomeric excess of the dihydroquinoxalinone containing trifluoromethyl can reach 99%. The method is simple, convenient and practical to operate, high in enantioselectivity and high in yield, and the reaction has green atom economyand is environmentally friendly.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for synthesizing chiral 3,4-dihydroquinazolinone through asymmetric hydrogenation of quinazolinone compound under catalysis of iridium
The invention provides a method for synthesizing chiral 3,4-dihydroquinazolinone through asymmetric hydrogenation of a quinazolinone compound under the catalysis of iridium, wherein in the formula defined in the specification, R is C1-6 alkyl or aryl containing a substituent group, R' is at least one selected from hydrogen, C1 alkyl, alkoxy and chlorine, and the substituent group is at least one selected from F, Cl, CF3, Ph, Me and MeO. According to the invention, with the method, the substrate range is wide, the synthesis of chiral 3,4-dihydroquinazolinone is diverse, and the enantiomeric excess can achieve 98%; and the method has characteristics of simple, convenient, practical and easily-performing operation, high yield, environmental protection, commercially available catalyst, mild reaction conditions and practical application value.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for synthesizing chiral amine by asymmetric hydrogenation of iridium-catalyzed pyrrole/indol [1,2-a] quinoxaline
ActiveCN109810109AHigh reactivityHigh enantioselectivityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsIridiumQuinoxaline
The invention provides a method for synthesizing chiral amine. Catalyzed pyrrole / indol [1,2-a] quinoxaline is prepared through asymmetric hydrogenation reaction, a catalyst is the complex of a metal precursor and chiral bisphosphine ligand of iridium, reaction activity and enantioselectivity are high, two kinds of hydrogenation products, i.e., an in situ protected hydrogenation product and a direct hydrogenation product, can be respectively or simultaneously obtained through regulating and controlling the added quantity and reaction time of acetic anhydride, a corresponding chiral tertiary amine compound is obtained, one or two kinds of high enantiomeric excess pure products can be obtained, and the enantiomeric excess of the pure products can be up to 97% at most. The method is simple, convenient, practical and easy in operation, high in yield and friendly to the environment, the catalyst can be commercially obtained, the reaction conditions are mild, and therefore, the method has a potential practical application value.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
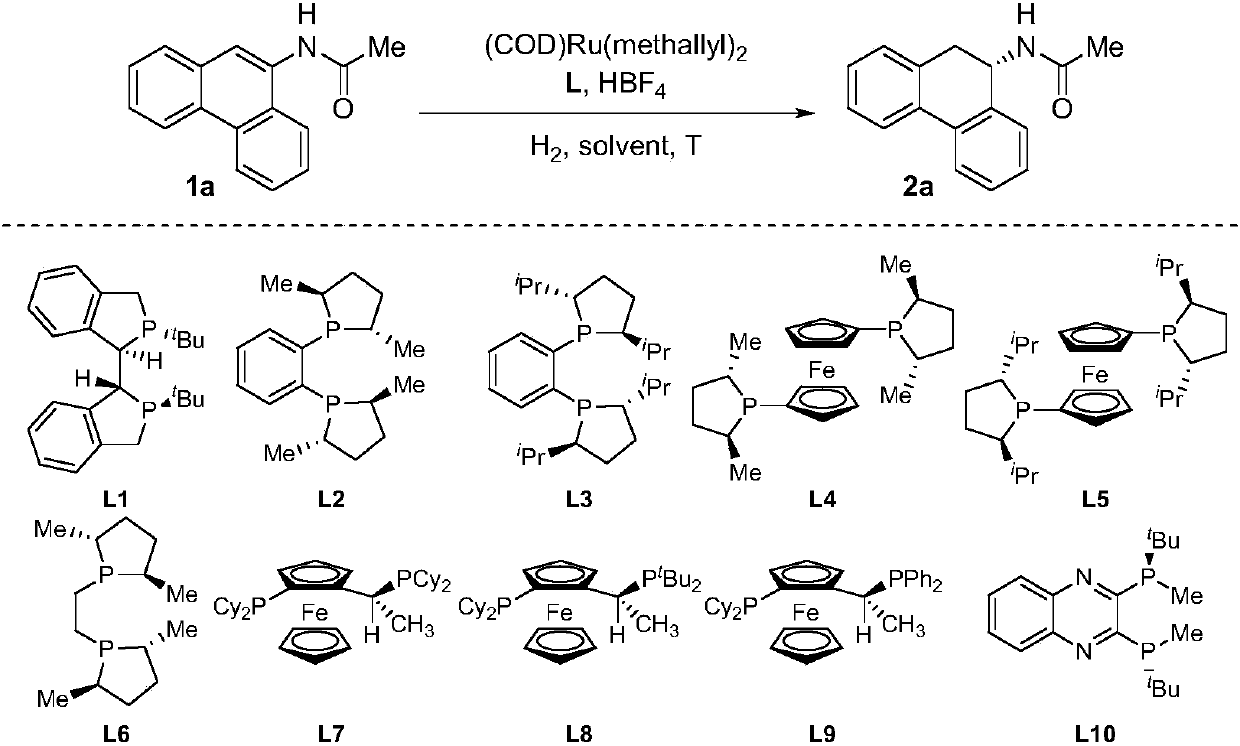
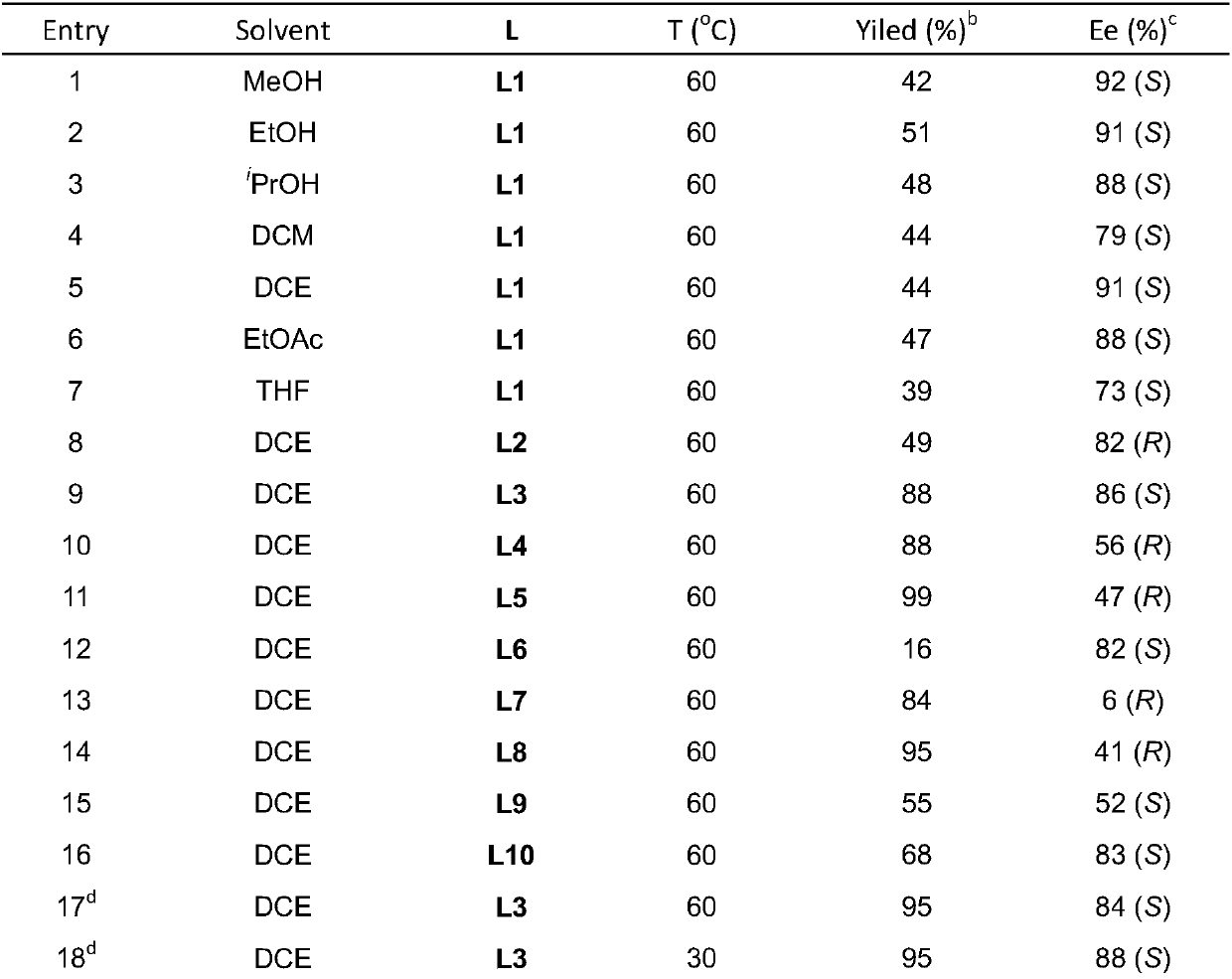
Method for synthesizing chiral tertiary amine by asymmetric hydrogenation of arylamine compound under catalysis of ruthenium
ActiveCN109574867AHigh reactivityHigh enantioselectivityOrganic compound preparationGroup 5/15 element organic compoundsAsymmetric hydrogenationDiphosphines
The invention discloses a method for synthesizing chiral tertiary amine by asymmetric hydrogenation of a 9-amide phenanthrene compound under catalysis of a ruthenium-diphosphine ligand. The method includes: adopting 2-5mol% of a ruthenium catalyst, and adding 4-10mol% of fluoboric acid for asymmetric hydrogenation of the 9-amide phenanthrene compound to obtain a corresponding chiral tertiary aminecompound, wherein the enantiomeric excess reaches 98%. The method is simple, convenient, practical and feasible in operation, high in yield, environmentally friendly and mild in reaction condition, the catalyst is commercially acquirable, and a potential practical application value is achieved.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for synthesizing chiral dihydro-6H-benzpyrole-[2,1-c][1,4]-benzodiazepine-6-ketone
InactiveCN103288831AHigh reactivityHigh enantioselectivityAsymmetric synthesesBenzodiazepineEnantiomer
The invention discloses a method for synthesizing chiral dihydro-6H-benzpyrole-[2,1-c][1,4]-benzodiazepine-6-ketone by asymmetric hydrogenation under catalysis of iridium. The reaction conditions are that the temperature is 0-50 DEG C; a solvent adopts a dichloromethane and methylbenzene mixed solvent in which the volume ratio of dichloromethane to methylbenzene is 1 to 2; the pressure is 13-50 atm; the proportion of a primer to a catalyst is 50 to 1; the catalyst is a complex consisting of (1,5-cyclooctadiene) iridium chloride dimer and diphosphine; an additive is morpholine trifluoroacetate or piperidine hydrochloride; a corresponding chiral dihydro product is obtained by hydrogenating seven-membered ring-shaped benzpyrole-[2,1-c][1,4]-benzodiazepine-6-ketone; enantiomer is excessive and can reach 96 percent. The method is easy to operate and practicable; raw materials are readily available; the enantioselectivity is high; the yield is high.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Preparation method of chiral beta-hydroxy acid ester compound
InactiveCN110330429AReduce pollutionHigh reactivityOrganic compound preparationOrganic chemistry methodsHydrogenBeta hydroxy acid
The invention discloses a preparation method of a chiral beta-hydroxy carboxylic ester compound. The method comprises the following steps: dissolving a dichlorophenyl ruthenium dimer, a ligand and analkaline additive in a solvent under the protection of inert gas, and stirring at room temperature to generate a catalyst in situ; dissolving the substrate, beta-carbonyl carboxylic ester, into the solvent, adding the prepared catalyst, and feeding hydrogen to carry out asymmetric catalytic hydrogenation reaction on the beta-carbonyl carboxylic ester, wherein the reaction conditions are as follows: the pressure is 10-100 bar, the reaction temperature is 0-200 DEG C, and the reaction time is 12-48 hours. The method has the advantages of high reaction activity and selectivity, mild hydrogenationreaction conditions, wide substrate application range and small environmental pollution in the reaction process, and is suitable for various beta-carbonyl carboxylic esters.
Owner:SHANGHAI SHINE HIGH INT TRADE CO LTD
Hydrogenation catalyst, preparation method and applications thereof, and phenol hydrogenation method
ActiveCN109420492AHigh catalytic activityImprove conversion rateOrganic compound preparationPreparation by hydrogenationActive componentHydrogenation reaction
The invention relates to a field of catalytic hydrogenation reactions, and discloses a hydrogenation catalyst, a preparation method and applications thereof, and a phenol hydrogenation method. The hydrogenation catalyst comprises a modified carrier and an active component loaded on the modified carrier. The active component is at least one of Pd, Rh, and Ru. The modified carrier is prepared by functionalizing a carrier by an amino-containing organic silanizing agent. The provided hydrogenation catalyst has high catalytic activity. In the hydrogenation reactions of phenols, the provided hydrogenation catalyst can still realize a high raw material conversion rate and product selectivity under mild conditions: low temperature, low pressure, and low hydrogen / phenols mole ratio, even if the content of the active components is low.
Owner:HUNAN CHANGLING PETROCHEM SCI & TECH DEV CO LTD
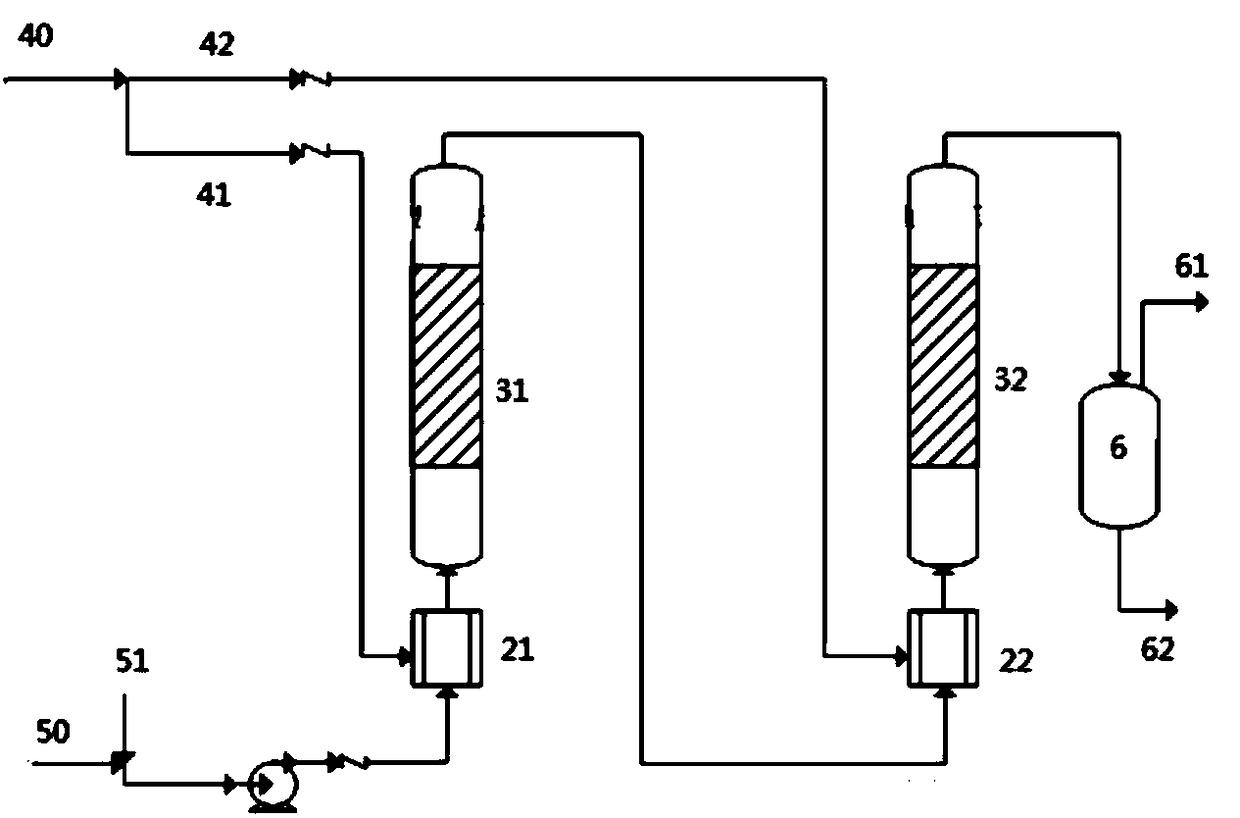
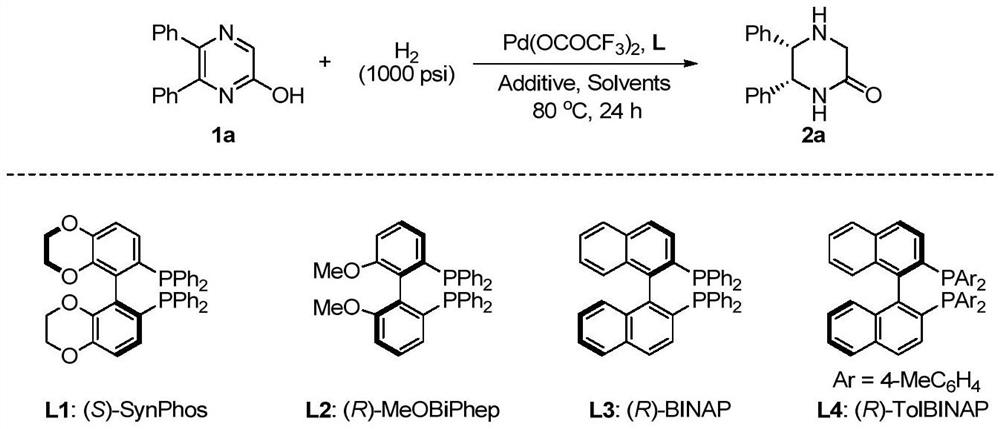
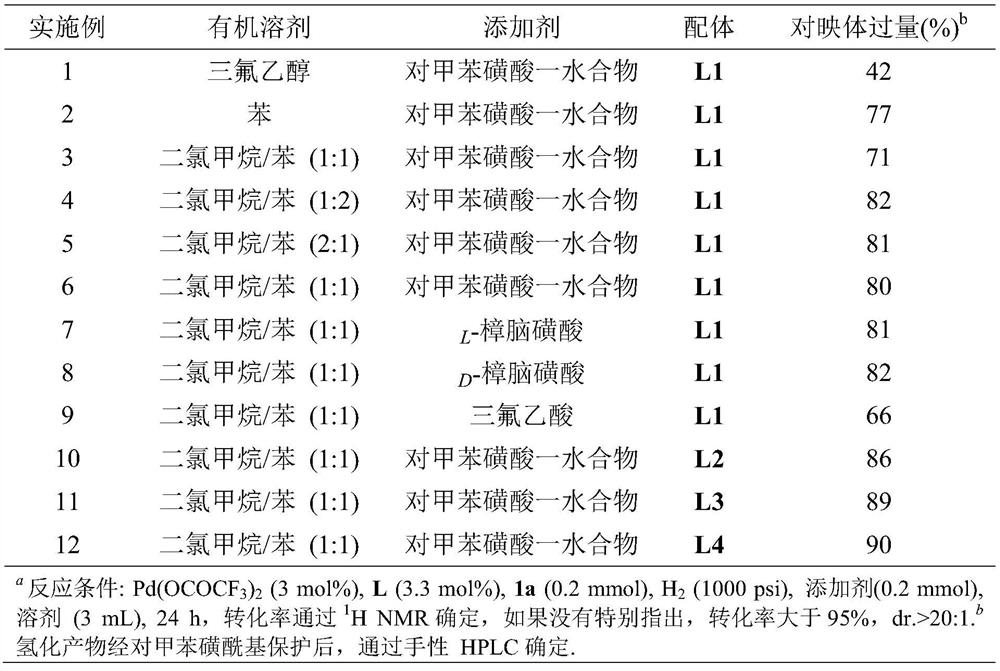
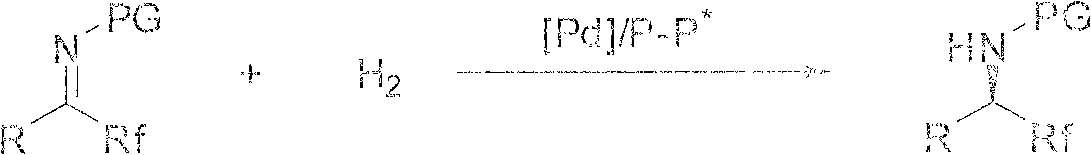
A method for palladium-catalyzed asymmetric hydrogenation of 2-hydroxypyrazine compounds to synthesize chiral lactams
The invention provides a palladium-catalyzed method for asymmetric hydrogenation of 2-hydroxypyrazine compounds to synthesize chiral lactams, wherein: R is C 1‑6 Alkyl or aryl containing substituents, the substituents are F, Cl, Br, CF 3 , Me, MeO, Et, n At least one of Pr. The invention has easy-to-obtain raw materials, high product enantioselectivity and good yield, and its enantiomeric excess can reach 90%. The catalyst is cheap and easy to obtain, and has good air stability, and provides an atom-economical and environmentally friendly route for the asymmetric hydrogenation of 2-hydroxypyrazine compounds to synthesize chiral lactams. At the same time, the present invention is simple and practical to operate, high in yield, environmentally friendly and green, mild in reaction conditions, and has potential practical application value.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for synthesizing chiral fluoroamine by palladium catalytic asymmetric hydrogenation
ActiveCN102336621BHigh reactivityHigh enantioselectivityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsSolventFluoroamine
The invention discloses a method for synthesizing chiral fluoroamine by palladium catalytic asymmetric hydrogenation, wherein, the used catalysis system comprises a palladium chiral diphosphite complex. The reaction is carried out under the following conditions that: the temperature is 0-50 DEG C, the solvent is a 2,2,2-trifluoroethyl alcohol, the pressure is 1-42 atm, the ratio of the substrate to the catalyst is 50 : 1, the used metal precursor is palladium trifluoroacetate, the used chiral ligand is a chiral diphosphite ligand. The catalyst is prepared by stirring the palladium metal precursor and the chiral diphosphite ligand in acetone at room temperature, and then carrying out vacuum condensation. According to the invention, by the hydrogenation of imine containing trifluoromethyl, corresponding chiral amine containing trifluoromethyl is obtained, the enantiomeric excess can reach to 94 %; by the hydrogenation of imine containing perfluoroalkyl, corresponding chiral amine containing perfluoroalkyl is obtained, and the enantiomeric excess can reach to 86 %. The present invention has the advantages of simple and practical operation, high enantioselectivitiy, good yield, green atom economy of the reaction, and no environmental pollution.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
A method for iridium-catalyzed asymmetric hydrogenation of quinoline-3-amine to synthesize chiral exocyclic amine
ActiveCN104710406BEasy to separateHigh reactivityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsAsymmetric hydrogenationDiphosphines
A method for synthesizing chiral exocyclic amine by iridium-catalyzed asymmetric hydrogenation of quinoline-3-amine, the catalytic system used is chiral diphosphorus complex of iridium. The reaction can be carried out under the following conditions, temperature: 25-70°C; solvent: a mixed solvent of toluene / tetrahydrofuran (V / V=3:1); pressure: 2-14 atmospheres; the ratio of substrate to catalyst is 25 / l; The catalyst is a complex of (1,5-cyclooctadiene) iridium chloride dimer and a chiral bisphosphine ligand. For quinoline-3-amine, the corresponding chiral exocyclic amine derivative can be obtained, and its enantiomeric excess value can reach 94%. The invention has the advantages of simple and practical operation, readily available raw materials, high enantioselectivity and good yield, and the reaction has green atom economy and is environmentally friendly.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for synthesizing chiral cyclic urea by asymmetric hydrogenation of 2-hydroxy pyrimidine compounds catalyzed by iridium
The invention provides a method for synthesizing chiral cyclic urea by asymmetric hydrogenation of 2-hydroxy pyrimidine compounds catalyzed by iridium. In the formula described below, R refers to alkyl or aryl of C1-C6; R' refers to aryl, heteroaryl or alkyl containing substituents, and the substituent contains at least one of F, Cl, CF3, Me and MeO, and the enantiomeric excess of the synthesizedchiral cyclic urea can reach 96%. The method is simple to operate and practical and easy to implement, high in the yield, environmental friendly, commercially available for catalysts and moderate in reaction condition and has potential practical application value.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Diphenylalkyl halide or diphenyl carboxylic acid and synthesis method thereof
ActiveCN101948368BProne to formatting reactionsReaction raw materials are cheapOrganic compound preparationHalogenated hydrocarbon preparationGrignard reagentSynthesis methods
The invention relates to diphenylalkyl halide or diphenyl carboxylic acid and a simple and convenient synthesis method thereof. The method comprises the following steps of: after a bromobenzene derivative and magnesium form a Grignard reagent, reacting the Grignard reagent and lactone, and opening loop to obtain 1,1-diphenyl-diol; reacting the 1,1-diphenyl-diol under the condition of a mixed solvent of an organic solvent and an acid to form diphenylenol; reacting the diphenylenol, a halogen or a halide and triphenylphosphine in the organic solvent to form diphenylalkenyl halogen; and reacting the diphenylalkenyl halogen and hydrogen to form diphenylalkyl halide, or reacting the diphenylenol and hydrogen to form diphenyl alcohol; and reacting the diphenyl alcohol and CrO3 under the action of the organic solvent and the acid to form diphenyl carboxylic acid. The reaction raw materials are cheap; the reaction conditions are mild; and substitutional diphenylalkyl halide or diphenyl carboxylic acid compounds with different benzene rings can be synthesized.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
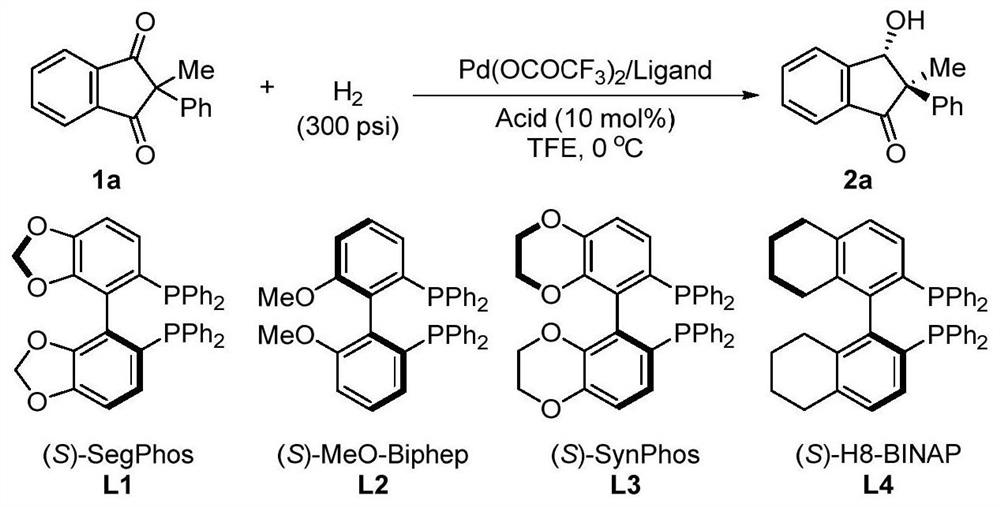
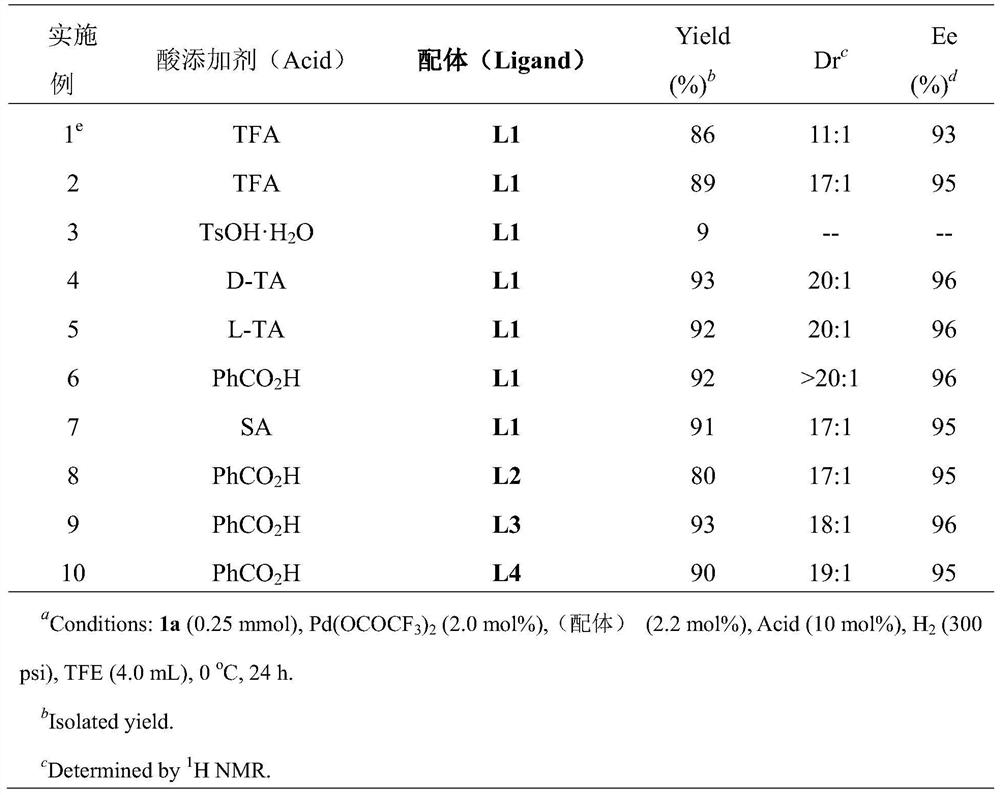
A method for palladium-catalyzed asymmetric hydrogenation of 1,3-diketones to synthesize β-hydroxy ketones
ActiveCN112028753BEasy to manufactureEasy to operateOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsDiketonePtru catalyst
A method for synthesizing chiral β-hydroxy ketones by desymmetrizing 1,3-diketones through palladium-catalyzed hydrogenation. Using 1-5mol% palladium catalyst and adding 10-100mol% acid to carry out asymmetric hydrogenation desymmetrization of 2-substituted 1,3-diketone compounds. The corresponding chiral β-hydroxy ketone compounds are obtained with an enantiomeric excess of up to 98%. The invention has the advantages of simple and practical operation, high yield, environmental friendliness, commercially available catalyst, mild reaction conditions and potential practical application value.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
A hydrogenation catalyst, its preparation method and application, and a method for hydrogenation reaction of phenols
ActiveCN109420492BThe hydrogenation reaction conditions are mildReduce the temperatureOrganic compound preparationPreparation by hydrogenationPtru catalystHydrogenation reaction
The invention relates to the field of catalytic hydrogenation reaction, and discloses a hydrogenation catalyst, a preparation method and application thereof, and a method for hydrogenation reaction of phenols. The hydrogenation catalyst comprises a modified carrier and an active component loaded on the modified carrier, the active component is selected from at least one of Pd, Rh and Ru, and the modified carrier is obtained by containing an amino group The organosilylating agent is used to functionalize the carrier. The hydrogenation catalyst of the present invention has relatively high catalytic activity, and in the hydrogenation reaction of phenolic compounds, the hydrogenation catalyst can still be used at low temperature, low pressure and low hydrogen phenol mole Ratio of mild conditions to achieve high conversion of raw materials and product selectivity.
Owner:HUNAN CHANGLING PETROCHEM SCI & TECH DEV CO LTD
Method for synthesizing chiral indoline through palladium catalyzed asymmetric hydrogenation of indole generated in situ
ActiveCN110790694AHigh reactivityHigh enantioselectivityOrganic-compounds/hydrides/coordination-complexes catalystsOrganic chemistry methodsPtru catalystPalladium catalyst
The invention discloses a method for synthesizing chiral indoline through palladium-catalyzed asymmetric hydrogenation of indole generated in situ. The method is characterized in that an indole compound generated in situ is subjected to asymmetric hydrogenation by using 1-5 mol% of a palladium catalyst and adding 1-2.0 equev acid to obtain a corresponding chiral indoline compound, wherein the enantiomeric excess can reach 96% at most. According to the invention, the method is simple and convenient to operate, practical, easy to implement, high in yield, environmentally friendly, available in catalyst business and mild in reaction condition, and has potential practical application value.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
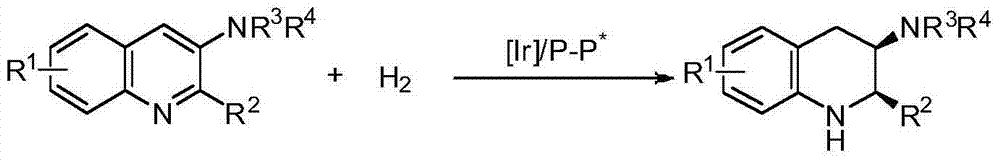
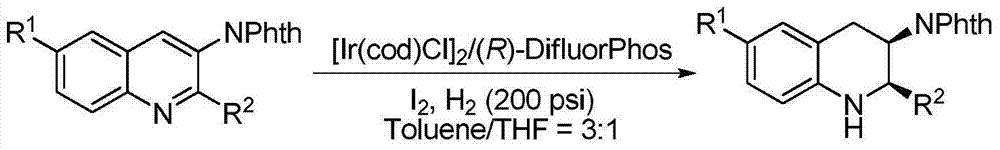
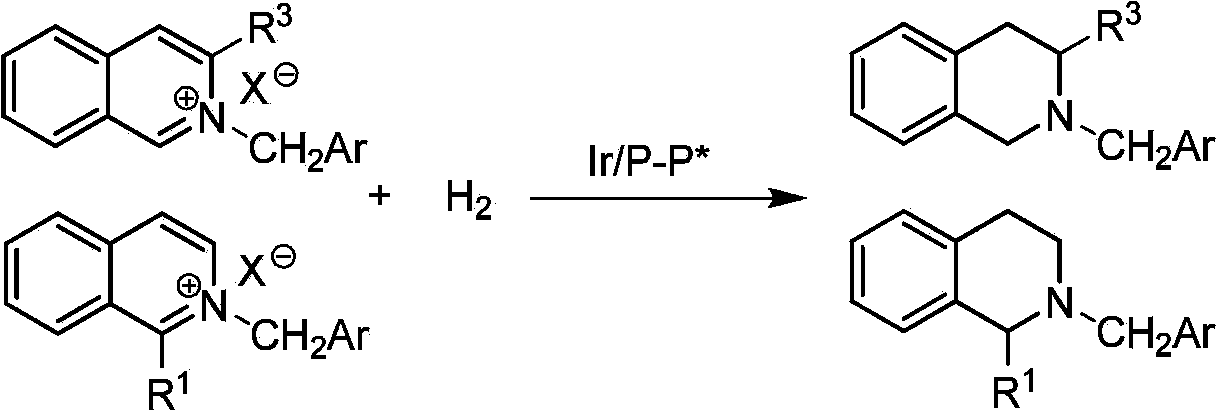
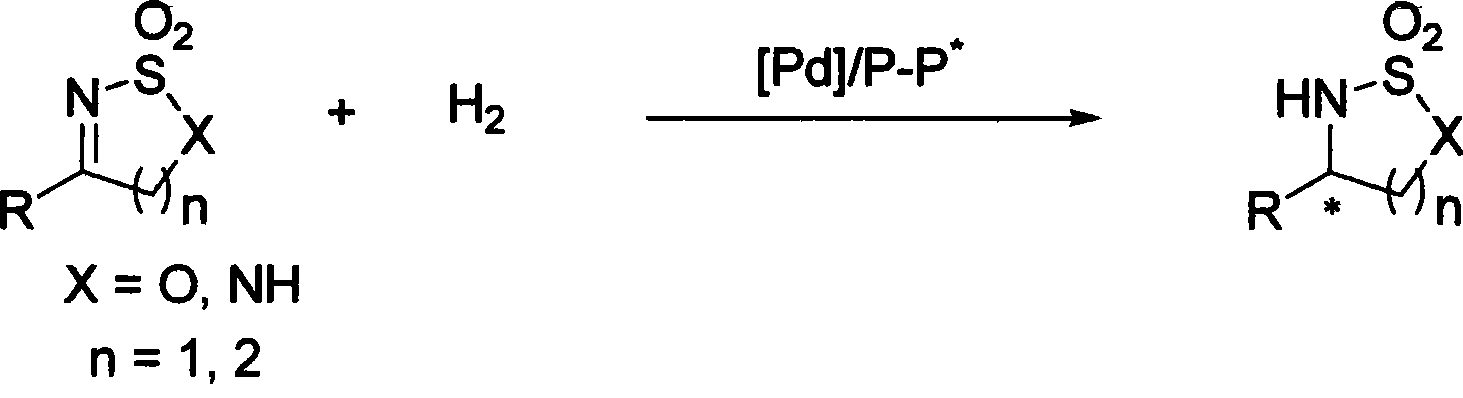
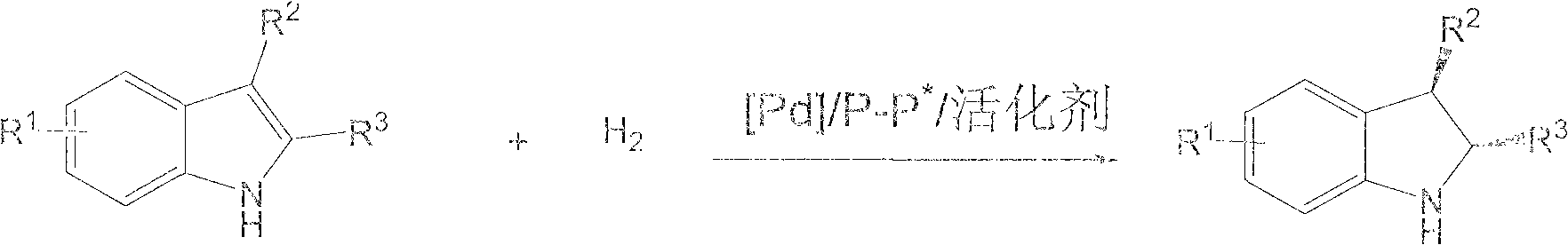
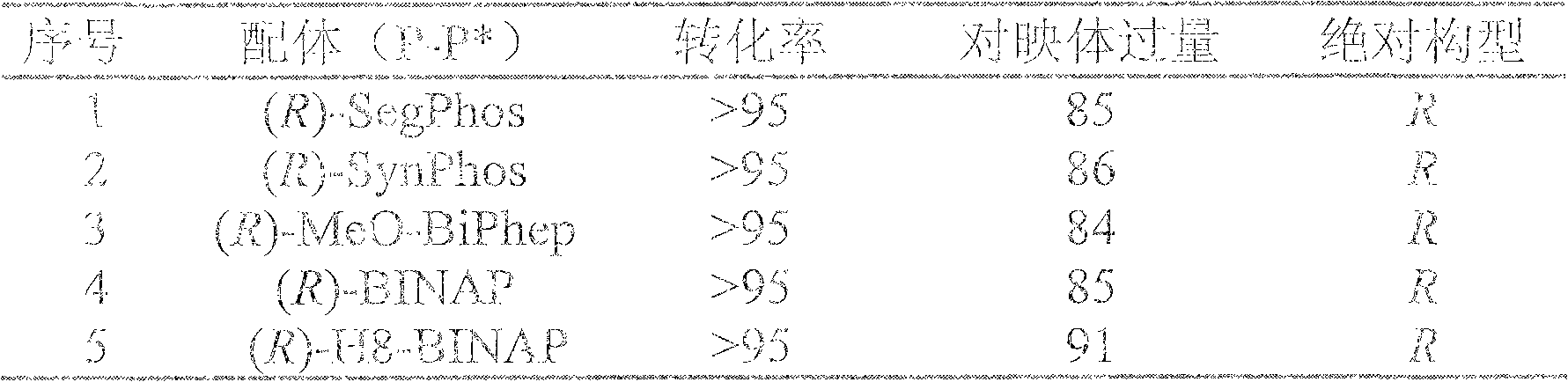
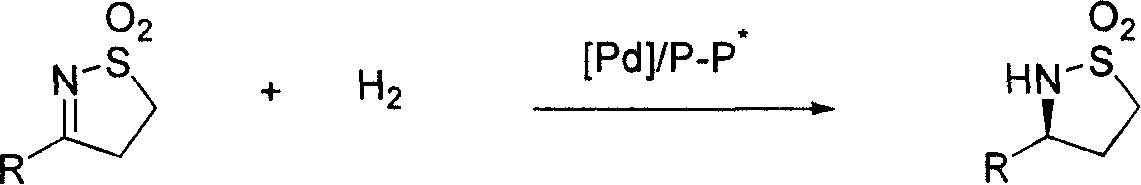
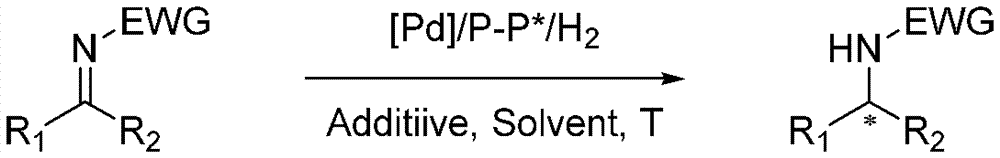
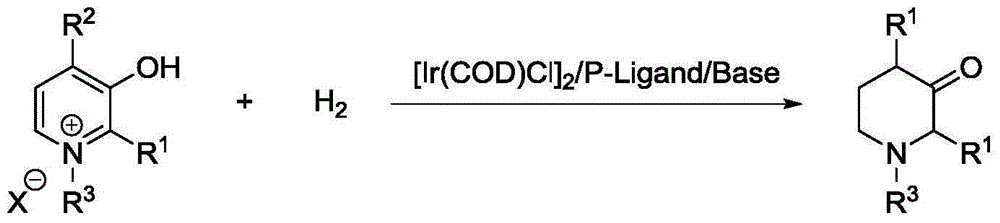
![Method for synthesizing chiral dihydro-5H-pyrrolo[2,1-c][1,4]-benzodiazepino-5-one Method for synthesizing chiral dihydro-5H-pyrrolo[2,1-c][1,4]-benzodiazepino-5-one](https://images-eureka.patsnap.com/patent_img/8721003a-0f8e-4840-b620-3c6dcd1a65ad/FDA0000138253650000011.png)
![Method for synthesizing chiral dihydro-5H-pyrrolo[2,1-c][1,4]-benzodiazepino-5-one Method for synthesizing chiral dihydro-5H-pyrrolo[2,1-c][1,4]-benzodiazepino-5-one](https://images-eureka.patsnap.com/patent_img/8721003a-0f8e-4840-b620-3c6dcd1a65ad/BDA0000138253660000021.png)
![Method for synthesizing chiral dihydro-5H-pyrrolo[2,1-c][1,4]-benzodiazepino-5-one Method for synthesizing chiral dihydro-5H-pyrrolo[2,1-c][1,4]-benzodiazepino-5-one](https://images-eureka.patsnap.com/patent_img/8721003a-0f8e-4840-b620-3c6dcd1a65ad/BDA0000138253660000051.png)
![Method for synthesizing chiral dihydro-6H-indolo[2,1-c][1,4]-benzodiazepine Method for synthesizing chiral dihydro-6H-indolo[2,1-c][1,4]-benzodiazepine](https://images-eureka.patsnap.com/patent_img/25bec905-6e0d-4049-a28e-94b0f58c6f5c/FDA0000138251660000011.png)
![Method for synthesizing chiral dihydro-6H-indolo[2,1-c][1,4]-benzodiazepine Method for synthesizing chiral dihydro-6H-indolo[2,1-c][1,4]-benzodiazepine](https://images-eureka.patsnap.com/patent_img/25bec905-6e0d-4049-a28e-94b0f58c6f5c/BDA0000138251670000021.png)
![Method for synthesizing chiral dihydro-6H-indolo[2,1-c][1,4]-benzodiazepine Method for synthesizing chiral dihydro-6H-indolo[2,1-c][1,4]-benzodiazepine](https://images-eureka.patsnap.com/patent_img/25bec905-6e0d-4049-a28e-94b0f58c6f5c/BDA0000138251670000051.png)
![Method for synthesizing chiral dihydro-5H-pyrrolo[2,1-c][1,4]-benzodiazepine Method for synthesizing chiral dihydro-5H-pyrrolo[2,1-c][1,4]-benzodiazepine](https://images-eureka.patsnap.com/patent_img/2ea8510d-18df-48d9-bf5f-addc10a7ec9b/FDA0000138247160000011.png)
![Method for synthesizing chiral dihydro-5H-pyrrolo[2,1-c][1,4]-benzodiazepine Method for synthesizing chiral dihydro-5H-pyrrolo[2,1-c][1,4]-benzodiazepine](https://images-eureka.patsnap.com/patent_img/2ea8510d-18df-48d9-bf5f-addc10a7ec9b/BDA0000138247170000011.png)
![Method for synthesizing chiral dihydro-5H-pyrrolo[2,1-c][1,4]-benzodiazepine Method for synthesizing chiral dihydro-5H-pyrrolo[2,1-c][1,4]-benzodiazepine](https://images-eureka.patsnap.com/patent_img/2ea8510d-18df-48d9-bf5f-addc10a7ec9b/BDA0000138247170000021.png)


![Method for synthesizing chiral amine by asymmetric hydrogenation of iridium-catalyzed pyrrole/indol [1,2-a] quinoxaline Method for synthesizing chiral amine by asymmetric hydrogenation of iridium-catalyzed pyrrole/indol [1,2-a] quinoxaline](https://images-eureka.patsnap.com/patent_img/5bff97ea-6960-4bfa-a783-ce0822efb6b8/FDA0001474915590000011.png)
![Method for synthesizing chiral amine by asymmetric hydrogenation of iridium-catalyzed pyrrole/indol [1,2-a] quinoxaline Method for synthesizing chiral amine by asymmetric hydrogenation of iridium-catalyzed pyrrole/indol [1,2-a] quinoxaline](https://images-eureka.patsnap.com/patent_img/5bff97ea-6960-4bfa-a783-ce0822efb6b8/FDA0001474915590000012.png)
![Method for synthesizing chiral amine by asymmetric hydrogenation of iridium-catalyzed pyrrole/indol [1,2-a] quinoxaline Method for synthesizing chiral amine by asymmetric hydrogenation of iridium-catalyzed pyrrole/indol [1,2-a] quinoxaline](https://images-eureka.patsnap.com/patent_img/5bff97ea-6960-4bfa-a783-ce0822efb6b8/FDA0001474915590000021.png)
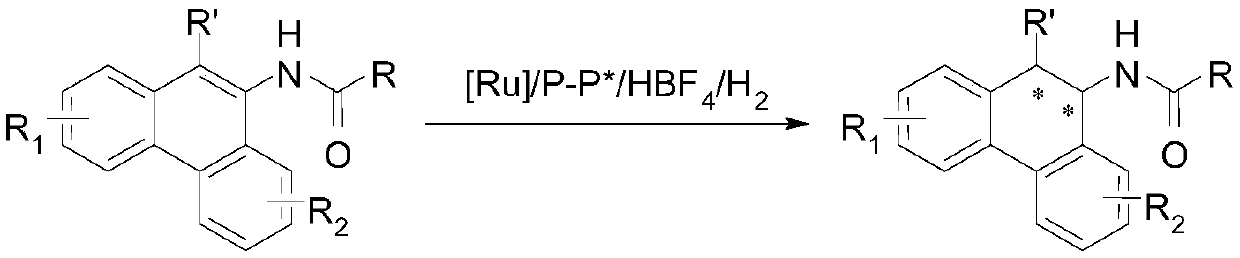
![Method for synthesizing chiral dihydro-6H-benzpyrole-[2,1-c][1,4]-benzodiazepine-6-ketone Method for synthesizing chiral dihydro-6H-benzpyrole-[2,1-c][1,4]-benzodiazepine-6-ketone](https://images-eureka.patsnap.com/patent_img/e6d181c0-4f2b-4590-96d9-77acc6e90e6f/FDA0000138255680000011.png)
![Method for synthesizing chiral dihydro-6H-benzpyrole-[2,1-c][1,4]-benzodiazepine-6-ketone Method for synthesizing chiral dihydro-6H-benzpyrole-[2,1-c][1,4]-benzodiazepine-6-ketone](https://images-eureka.patsnap.com/patent_img/e6d181c0-4f2b-4590-96d9-77acc6e90e6f/BDA0000138255690000021.png)
![Method for synthesizing chiral dihydro-6H-benzpyrole-[2,1-c][1,4]-benzodiazepine-6-ketone Method for synthesizing chiral dihydro-6H-benzpyrole-[2,1-c][1,4]-benzodiazepine-6-ketone](https://images-eureka.patsnap.com/patent_img/e6d181c0-4f2b-4590-96d9-77acc6e90e6f/BDA0000138255690000051.png)