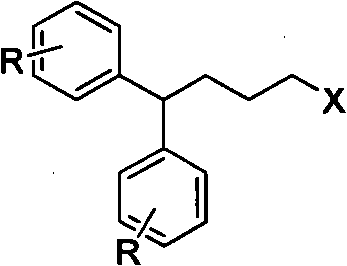
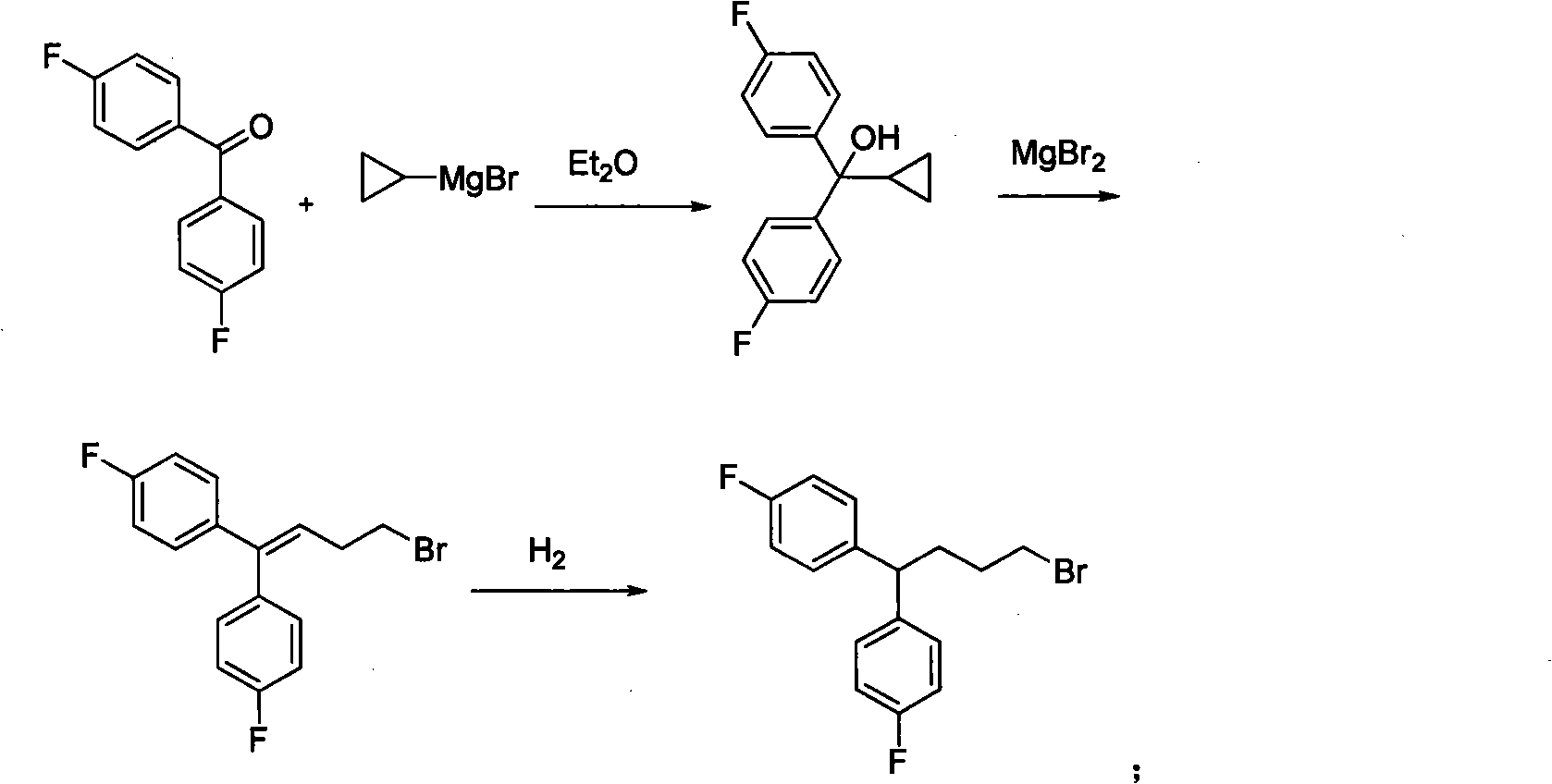
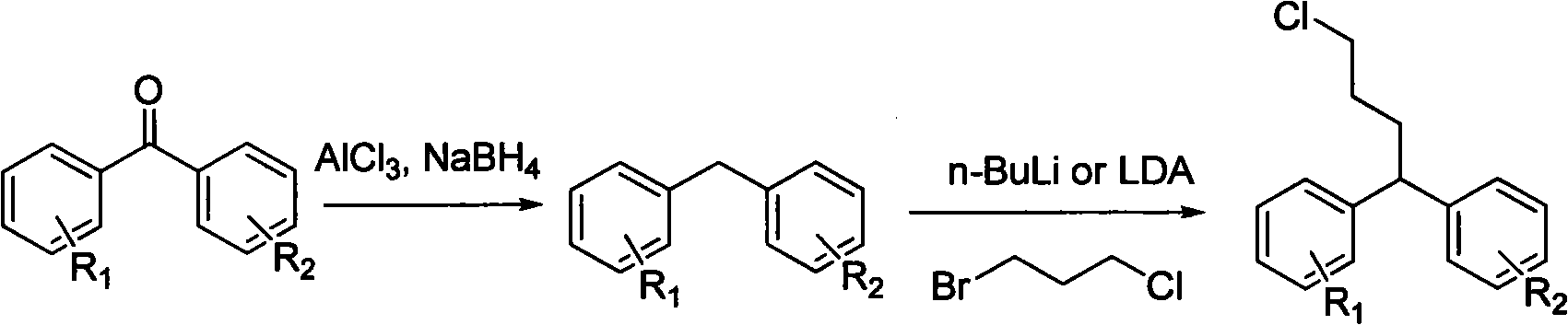
Diphenylalkyl halide or diphenyl carboxylic acid and synthesis method thereof
A technology of diphenyl alkyl halide and diphenyl carboxylic acid, which is applied in the field of simple synthesis, and can solve the problems of high price, troublesome synthesis, poor repeatability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: the preparation of substituted 4,4-diphenylbutyl bromide (4)
[0046]
[0047] General experiment operation:
[0048] A dry 250mL three-necked flask was connected with a reflux condenser, and magnesium chips (2.32g, 97mmol) and several grains of iodine were added, the vacuum was changed three times, and 20mL of dry THF was added. The bromobenzene derivative (100 mmol) and 20 mL of dry THF were added to the dropping funnel, and a quarter of the mixture was first added to initiate the Grignard reaction (heating if necessary). After the iodine color disappears, continue to react for half an hour and slowly add the remaining mixture. After the magnesium flakes disappeared, continue to react for half an hour and then slowly drop in 1,4-butyrolactone (3.44g, 40mmol) and 20mL of dry THF, a little exotherm. After dripping, heat to reflux for about 3 hours, stop the reaction, quench with 1M hydrochloric acid, extract three times with ethyl acetate, wash the or...
Embodiment 24
[0079] Embodiment 24, the preparation of 4-diphenyl 1-butyric acid compound (6)
[0080]
[0081] Add 262mg (1mmol) into a 100mL three-necked flask, add 10ml of acetone, cool to zero, add dropwise the pre-configured Jones reagent, a precipitate is formed, the color of the solution changes from green to orange red, and after the color remains unchanged for half an hour, add isopropyl Alcohol, the solution turns green again. After the solid was filtered out with suction, spin-dried, extracted with ether, washed with saturated sodium bicarbonate and sodium chloride, and dried over anhydrous sodium sulfate. Spin-dried and passed through the column to obtain 285 mg (>99%) of oily substance.
[0082] 4,4-bis(4-fluorophenyl)butanoic acid (6)
[0083] 1 H NMR (300MHz, CDCl 3 )δ7.13-7.18 (m, 4H), 694-7.00 (m, 4H), 3.92 (t, J=6.0Hz, 1H), 2.30-2.33 (m, 2H); LREI-MS m / z: 376 [M] + , 258, 229, 216, 203, 183, 125, 109, 75, 57, 43.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com