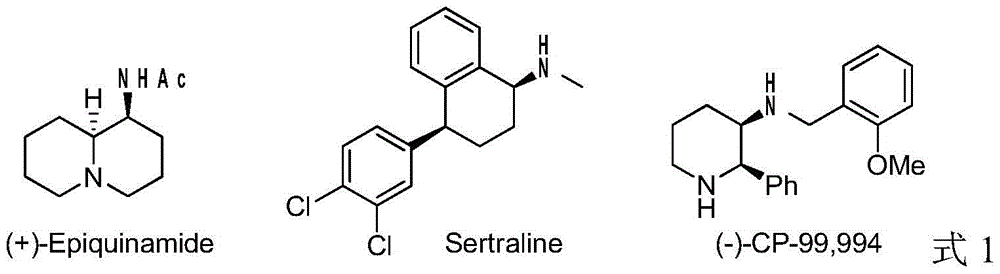
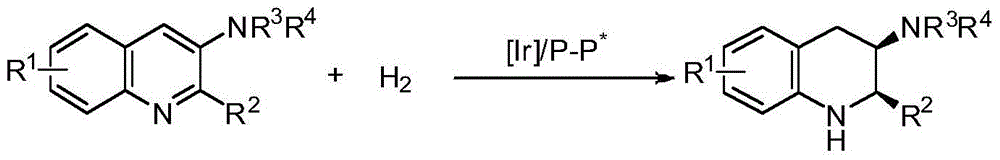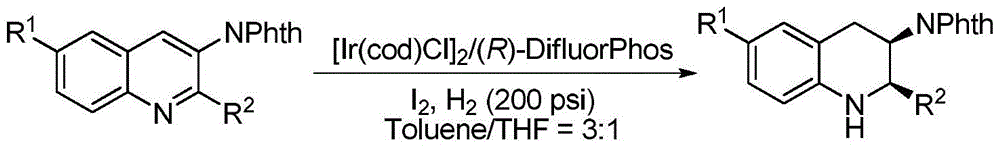A method for iridium-catalyzed asymmetric hydrogenation of quinoline-3-amine to synthesize chiral exocyclic amine
A technology of chiral exocyclic amines and catalyzed quinoline, applied in chemical instruments and methods, catalytic reactions, physical/chemical process catalysts, etc., can solve problems such as strong coordination ability, low activity of aromatic amines, catalyst poisoning, etc., to achieve The effect of complete reaction, convenient preparation, high reactivity and enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Synthesis of various chiral exocyclic amine compounds by iridium-catalyzed asymmetric hydrogenation of quinoline-3-amine
[0033] In a glove box filled with nitrogen, the reaction of (1,5-cyclooctadiene) iridium chloride dimer (0.0020 mmol) and chiral ligand (R)-DifluorPhos (0.0044 mmol) Add 1mL mixed solvent toluene / ditetrahydrofuran (v / v=3:1) into the bottle, stir the resulting solution at room temperature for 10-30 minutes, then transfer the prepared catalyst to another bottle containing the raw material quinoline-3 - In the reaction vials of amine (0.10 mmol) and iodine (0.0050 mmol), share 3 mL of solvent mixture solvent toluene / tetrahydrofuran (v / v=3:1). Stir at room temperature for 10-30 minutes, put the reaction bottle into a stainless steel autoclave, feed hydrogen gas at 200psi, and react at room temperature (or 45°C) for 18 hours. After the reaction, hydrogen gas was released slowly, the solvent was removed by a rotary evaporator, and the pure pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com