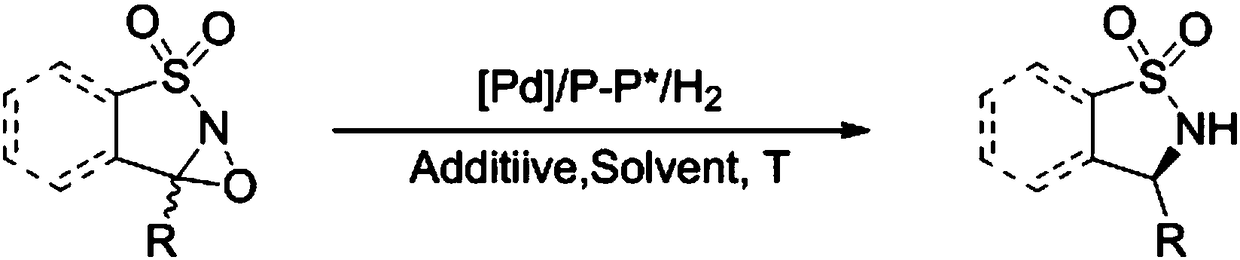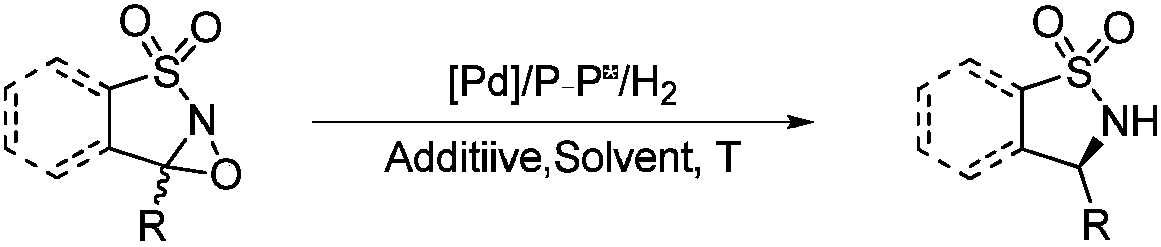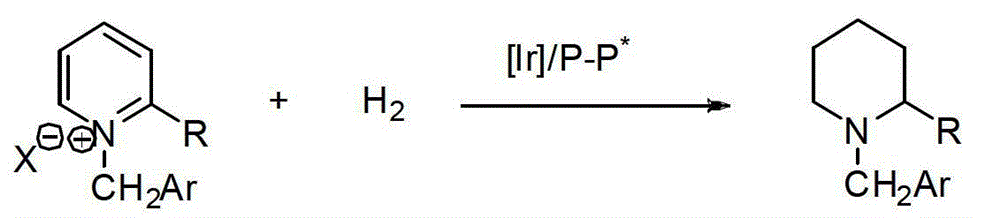Patents
Literature
32results about How to "Pure high enantiomeric excess" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
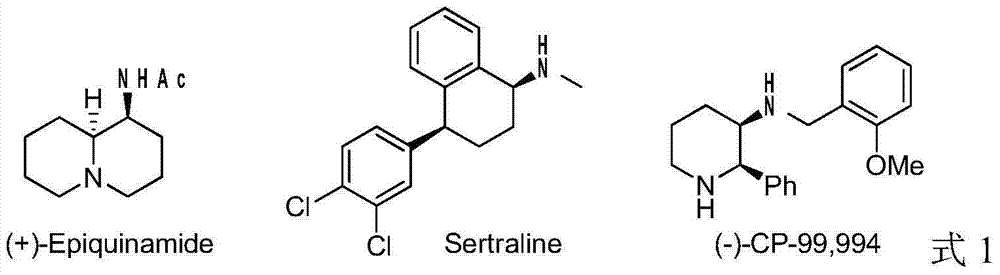
Method for synthesizing chiral cyclic amine through catalyzing asymmetric hydrogenation of quinolin-3-amine by iridium
ActiveCN104710406AEasy to separateHigh reactivityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsDiphosphinesIridium chloride
A catalysis system used by a method for synthesizing chiral cyclic amine through catalyzing asymmetric hydrogenation of quinolin-3-amine by iridium is a chiral diphosphine complex of iridium. A reaction is carried out under the following conditions: the temperature is 25-70DEG C; a solvent is a toluene / tetrahydrofuran mixed solvent (V / V=3:1); the pressure is 2-14atm; a ratio of a substrate to a catalyst is 25:1; and the catalyst is a (1,5-cyclooctadiene)iridium chloride dimer and chiral diphosphine ligand complex. A corresponding chiral cyclic amine derivative is prepared from quinolin-3-amine, and the enantiomeric excess value can reach 94%. The method has the advantages of simple and practical operation, easily available raw material, high enantioselectivity, good yield, and green, atom-economic and environmentally-friendly reaction.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
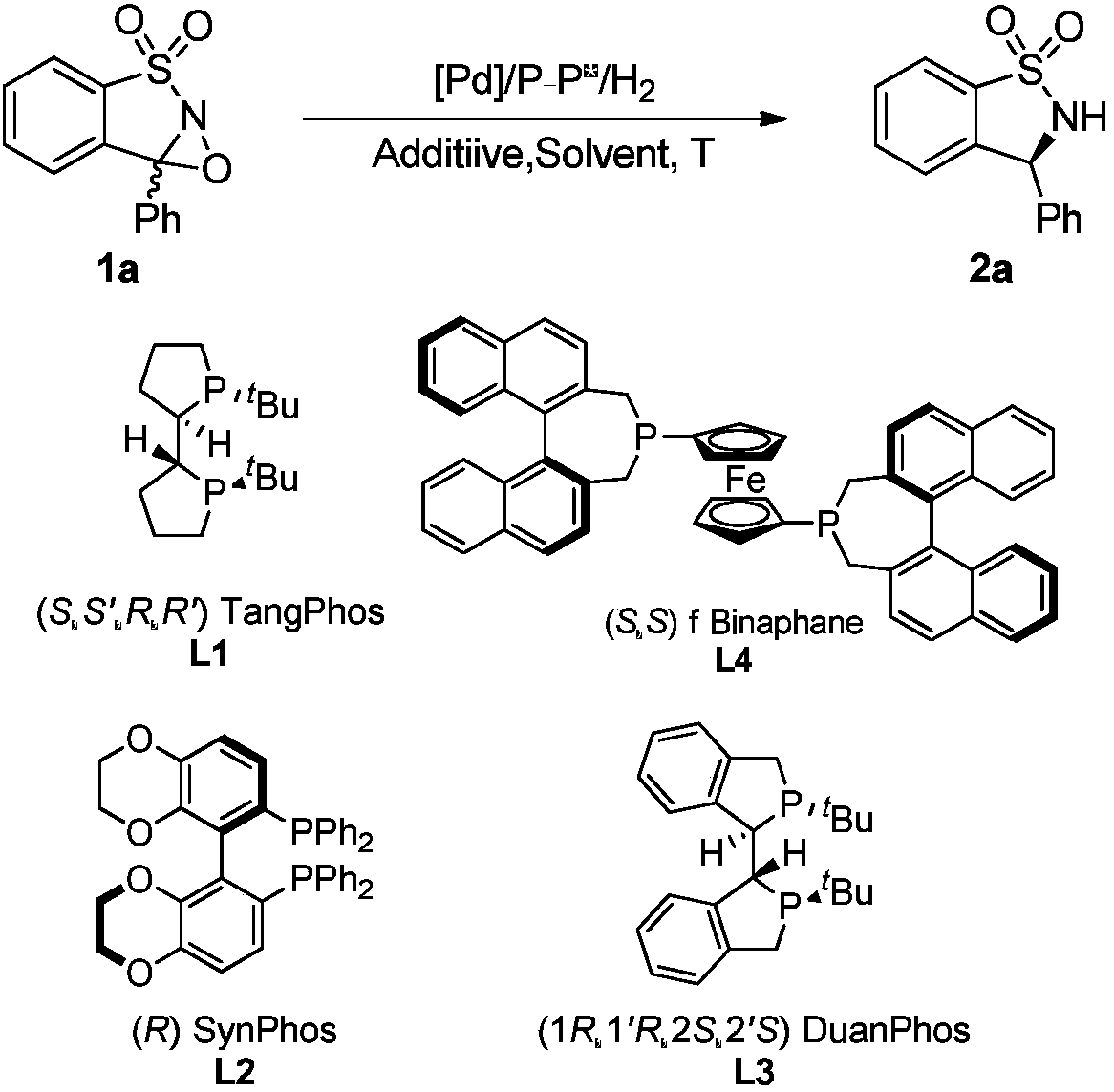
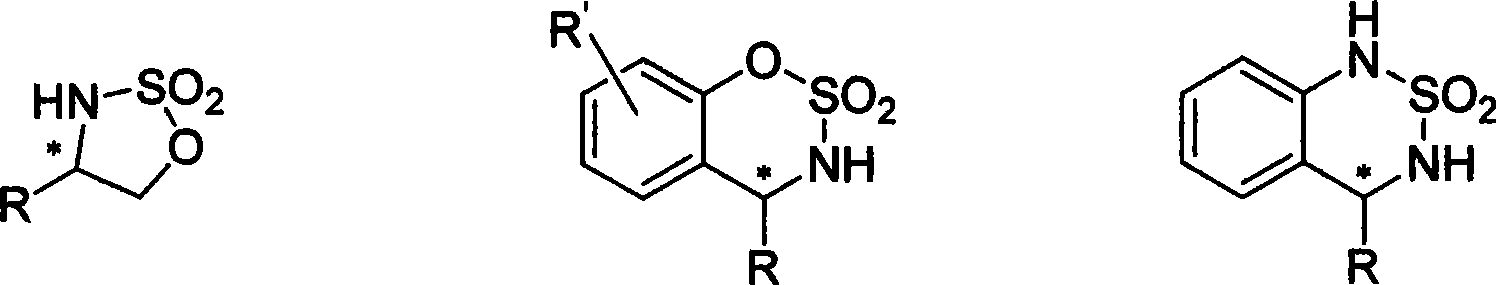
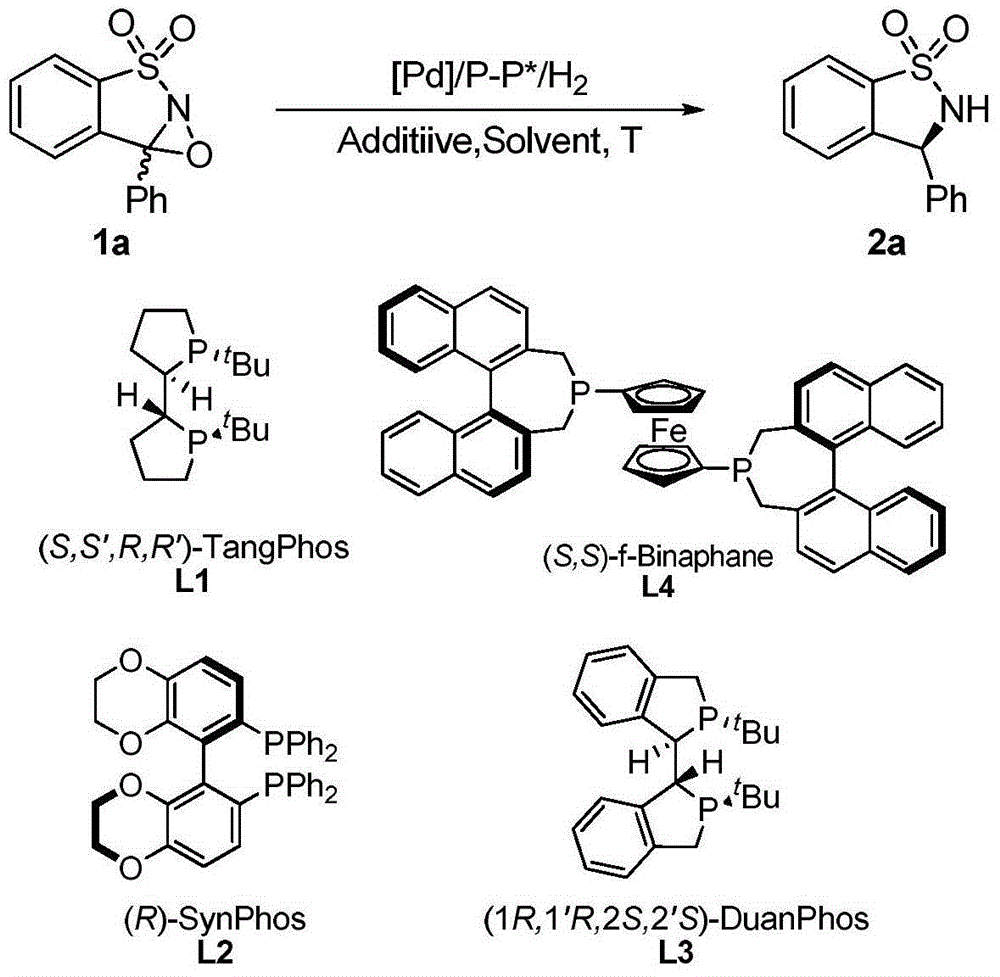
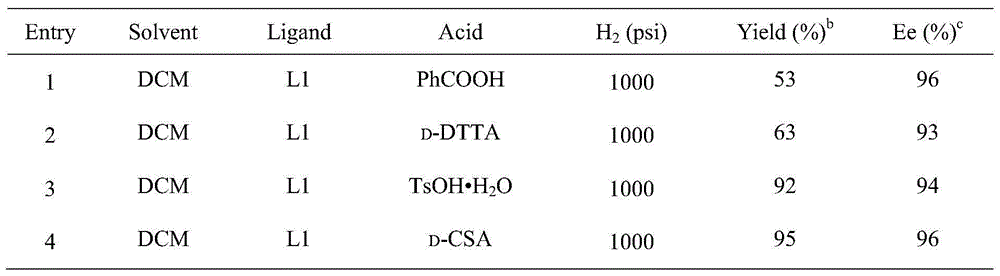
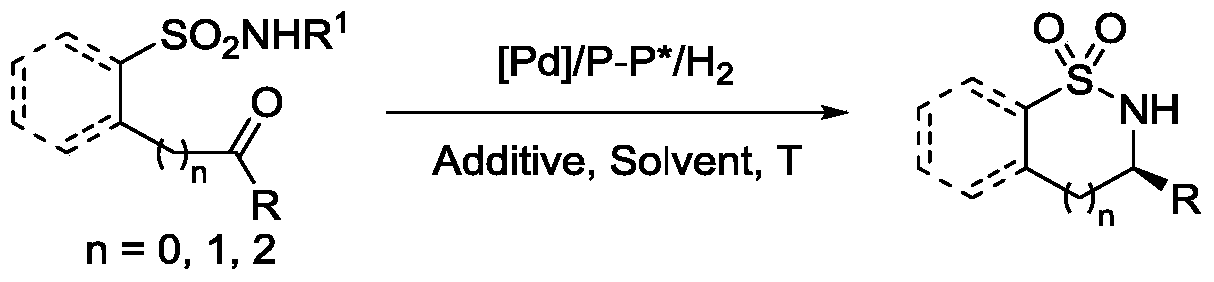
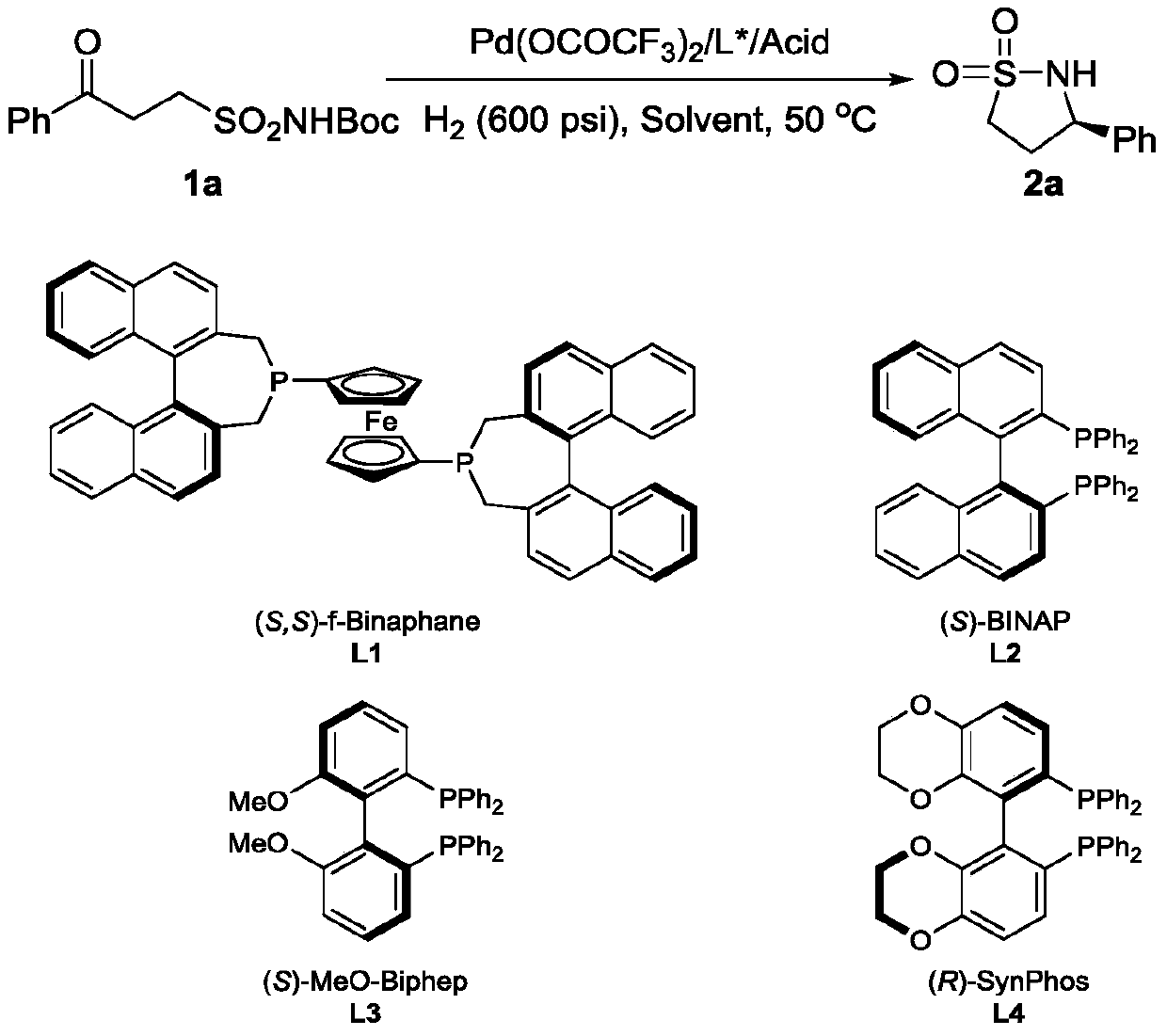
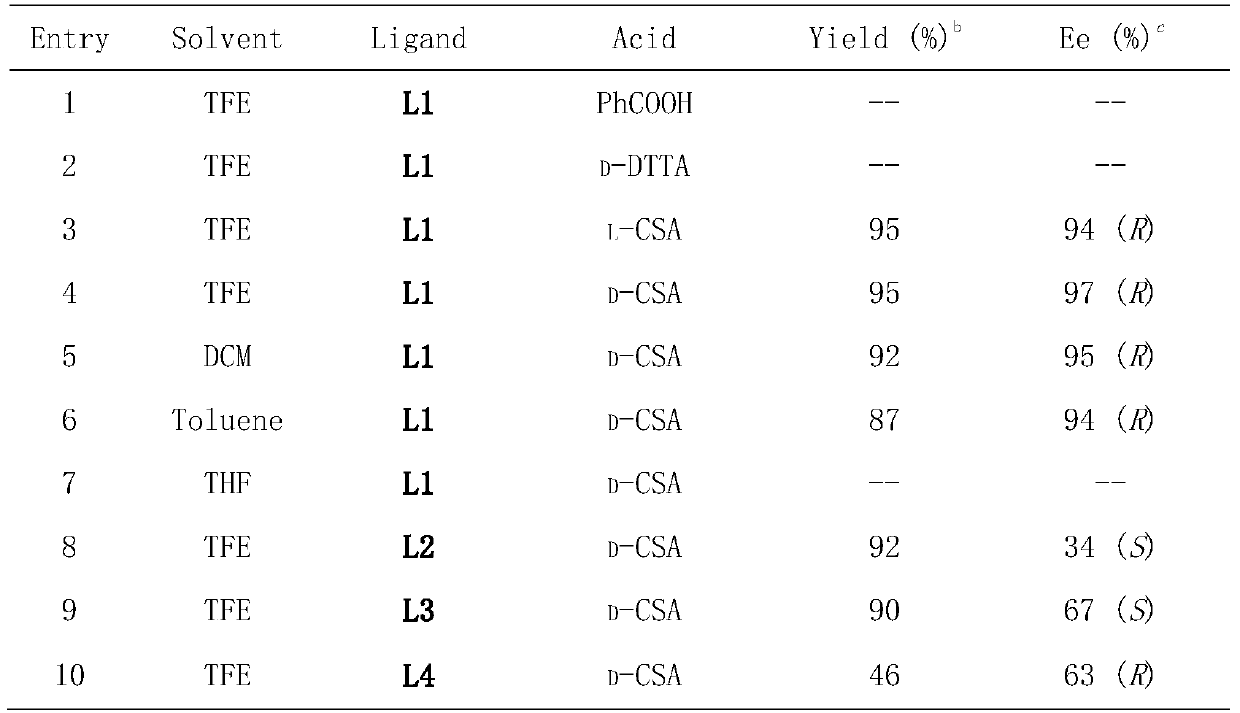
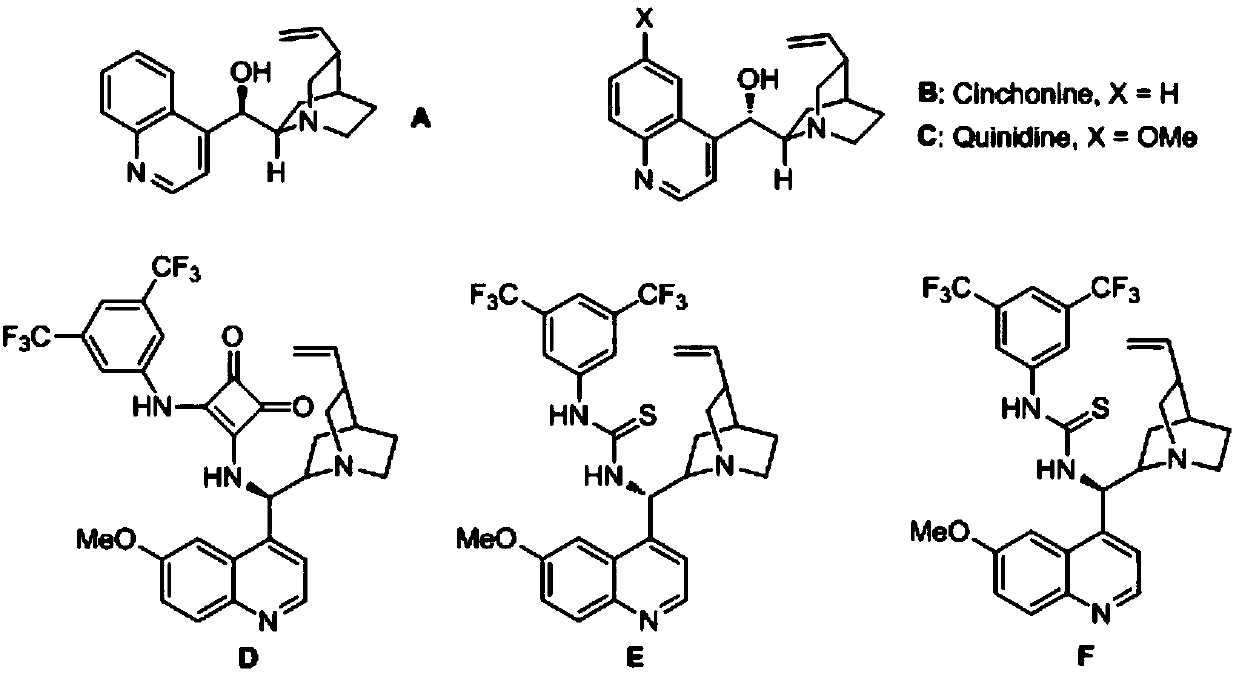
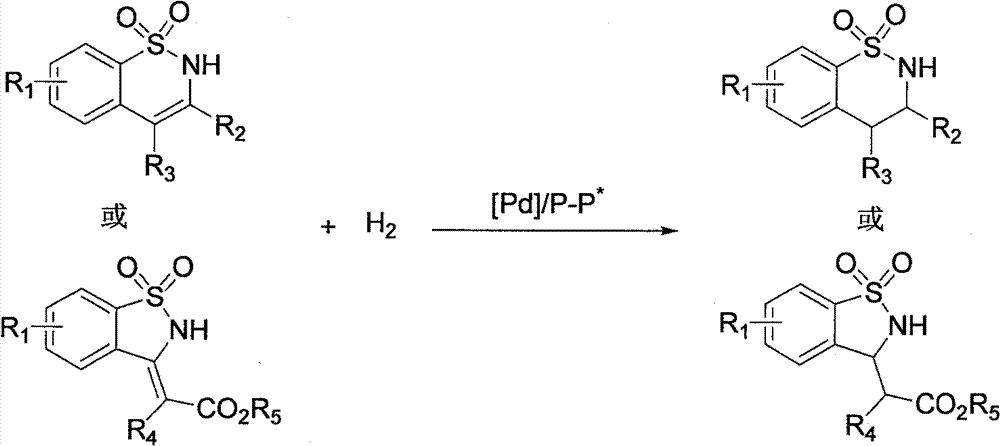
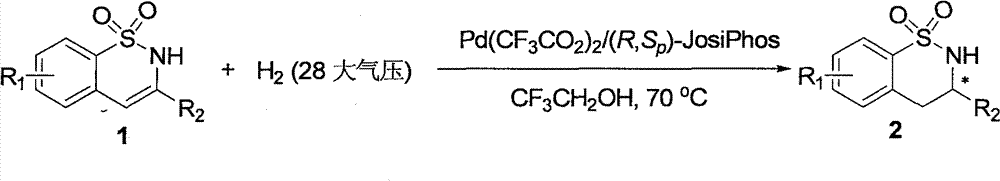
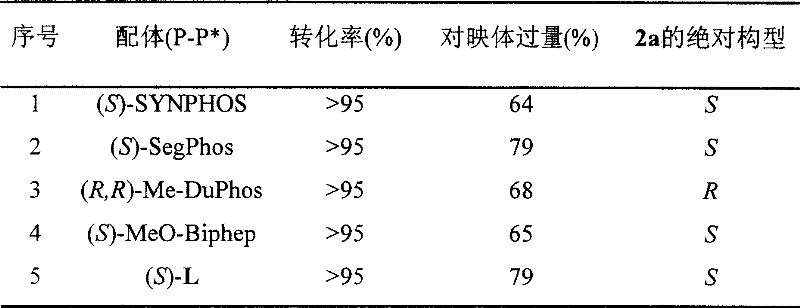
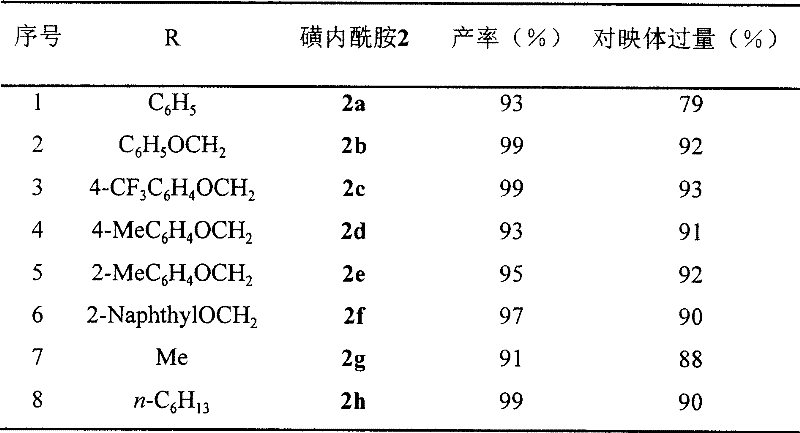
Method for synthesizing chiral sultam containing hetero atom unsymmetrical hydrogenation using Pd as catalyst
InactiveCN101423504AHigh reactivity and enantioselectivityRespond completelyOrganic-compounds/hydrides/coordination-complexes catalystsAsymmetric synthesesSolvent2,2,2-Trifluoroethanol
The invention provides a method for synthesizing chiral sultam containing hetero atoms by palladium-catalyzed asymmetric hydrogenation, wherein the catalyst is a chiral diphosphine ligand of palladium. The reaction is carried out at a temperature of between 25 and 60 DEG C and at a pressure of 40 atmosphere for 12 hours in solvent of 2, 2, 2-trifluoroethanol. Sulfonyl oxathiazinane heterocycle five-membered cyclic imide is hydrogenated to obtain corresponding chiral sultam containing oxathiazinane heterocycles with enantiomeric which excessively reaches 97 percent; and benzo N-sulfonyl oxathiazinane heterocycle six-membered cyclic imide is hydrogenated to obtain corresponding chiral sultam containing oxathiazinane heterocycles with enantiomeric which excessively reaches 99 percent; and benzo N-sulfonyl benzothiazinane heterocycle six-membered cyclic imide is hydrogenated to obtain corresponding chiral sultam containing benzothiazinane heterocycles with enantiomeric which excessively reaches 98 percent. The method has the advantages of simple and practical operation, high enantiomeric selection and good yield, and the reaction has green atomic economy and is friendly to environment.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
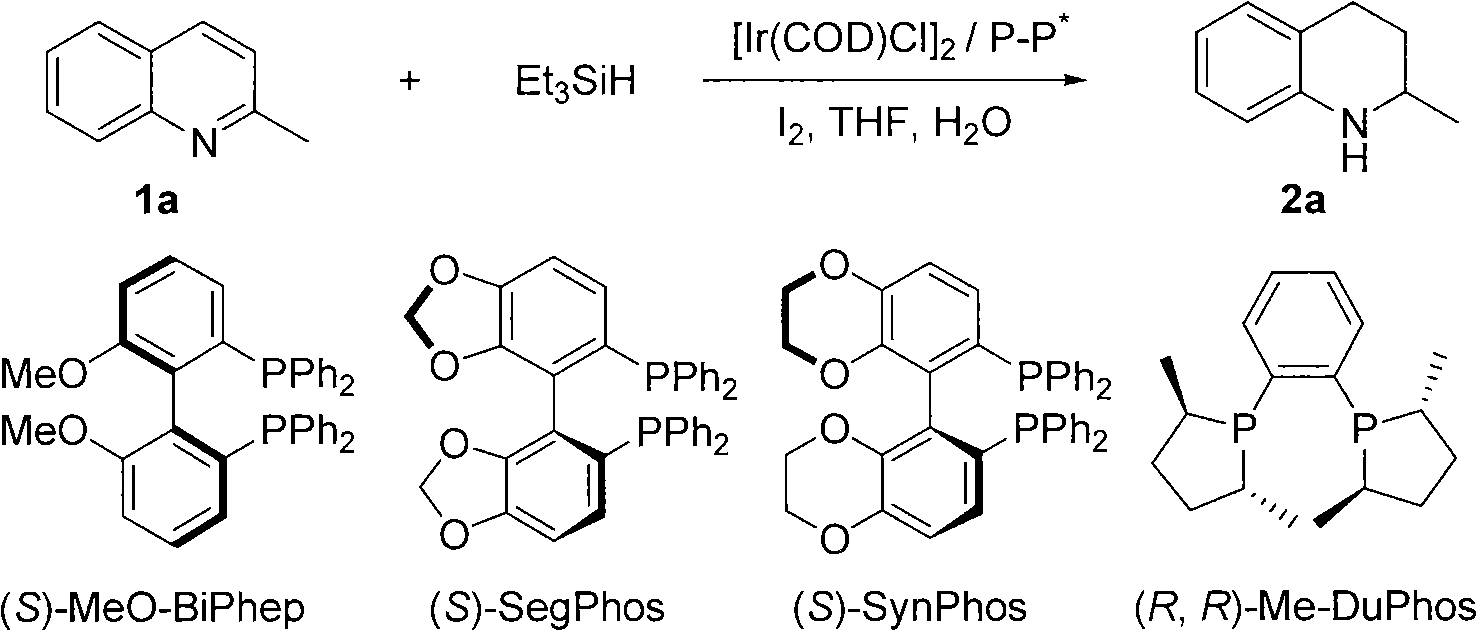
Method for synthesizing derivatives of chiral tetrahydroquinoline by catalyzing asymmetric hydrosilylation with iridium
InactiveCN101845016AReduce usageHigh reactivityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsIridiumQuinoline
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for synthesizing chiral phosphoramidate by carrying out asymmetric hydrogenation on palladium-catalyzed imine phosphate
InactiveCN106866730AHigh reactivityHigh enantioselectivityGroup 5/15 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsPhosphateAsymmetric hydrogenation
The invention discloses a palladium-catalyzed method for asymmetric hydrogenation of imine phosphonate to synthesize chiral amino phosphonate. The catalyst system used is palladium chiral bisphosphorus complex. Asymmetric hydrogenation of a series of imine phosphonates can give the corresponding chiral amino phosphonates with an enantiomeric excess of 99%. The invention is simple and practical to operate, the catalyst is commercially available, and the reaction conditions are mild. In addition, the synthesis of chiral aminophosphonates by asymmetric hydrogenation has high enantioselectivity and good yield, and the reaction has green atom economy and is environmentally friendly.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for synthesizing chiral dihydro-5H-pyrrolo[2,1-c][1,4]-benzodiazepino-5-one
InactiveCN103288829AHigh reactivityHigh enantioselectivityAsymmetric synthesesMorpholineDiphosphines
The invention relates to a method for synthesizing chiral dihydro-5H-pyrrolo[2,1-c][1,4]-benzodiazepino-5-one by iridium-catalyzed asymmetric hydrogenation. The reaction conditions are as follows: the temperature is 20-50 DEG C; the solvent is a dichloromethane-toluene mixed solvent (V / V=1:2); the pressure is 13-50 atmospheric pressures; the ratio of the substrate to the catalyst is 50 / 1; the catalyst is a complex of (1,5-cyclooctadiene)iridium chloride dimer and diphosphine ligand; and the additive is morpholine trifluoroacetate or piperidine hydrochloride. Seven-element cyclic pyrrolo[2,1-c][1,4]-benzodiazepino-5-one is hydrogenated to obtain the corresponding chiral dihydro product, and the enantiomeric excess can reach 96%. The invention is simple and practical to operate, and has the advantages of accessible raw materials, favorable selectivity for antipode, high yield, atomic economical efficiency for reaction, and environment friendliness.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for synthesizing chiral cyclohexanediamine through asymmetric hydrogenation of palladium-catalyzed quinoline-3-amine
ActiveCN105017150AEasy to separateHigh reactivityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsHydrogen pressureReaction temperature
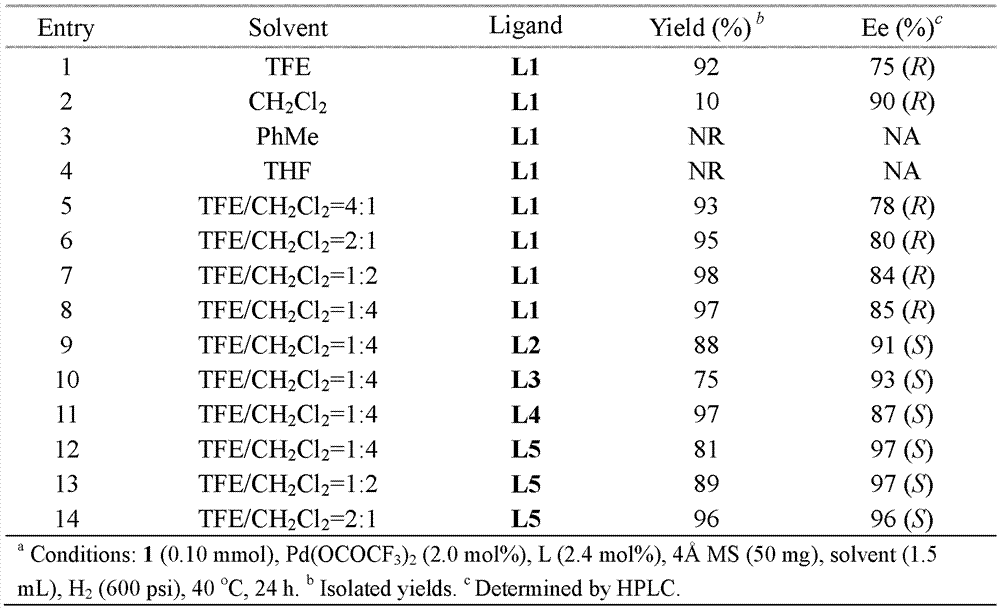
The invention discloses a method for synthesizing chiral cyclohexanediamine through asymmetric hydrogenation of palladium-catalyzed quinoline-3-amine. The method is characterized in that bronsted acid is used as an activating agent, and a catalytic system used in the method is a chiral biphosphine complex of palladium. The reaction can be carried out under the following conditions: the reaction temperature ranges from 60 DEG C to 80 DEG C; dichloromethane is used as a reaction reagent; the hydrogen pressure is 20-70 atmospheres; the molar ratio of a substrate to a catalyst is 20:1; palladium trifluoroacetate (Pd(OCOCF3)2) is used as a metal precursor; a chiral biphosphine ligand is used as a chiral ligand; and trifluoroacetic acid (CF3CO2H) is used as an activating agent. Corresponding chiral cyclohexanediamine derivatives can be prepared from quinoline-3-amine, and the enantiomeric excess can be up to 90%. The method is simple and convenient to operate, available in raw material, good in enantioselectivity, high in yield, capable of realizing green atom economy in reaction and environment-friendly.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
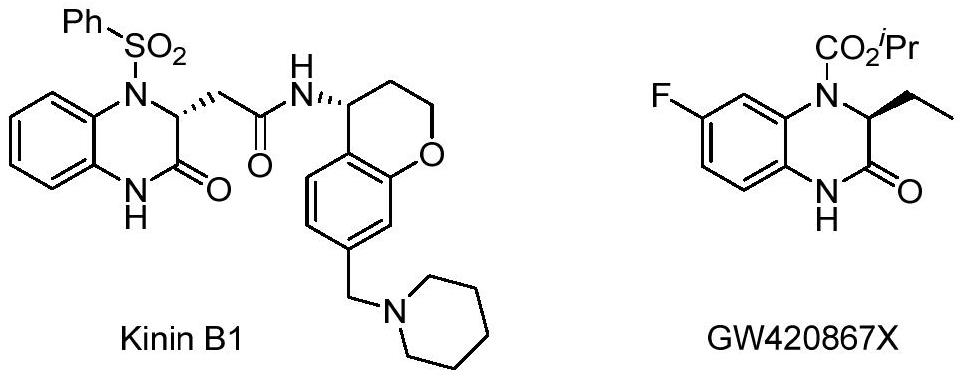
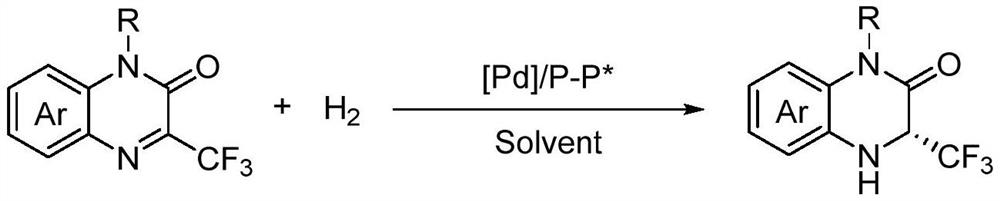
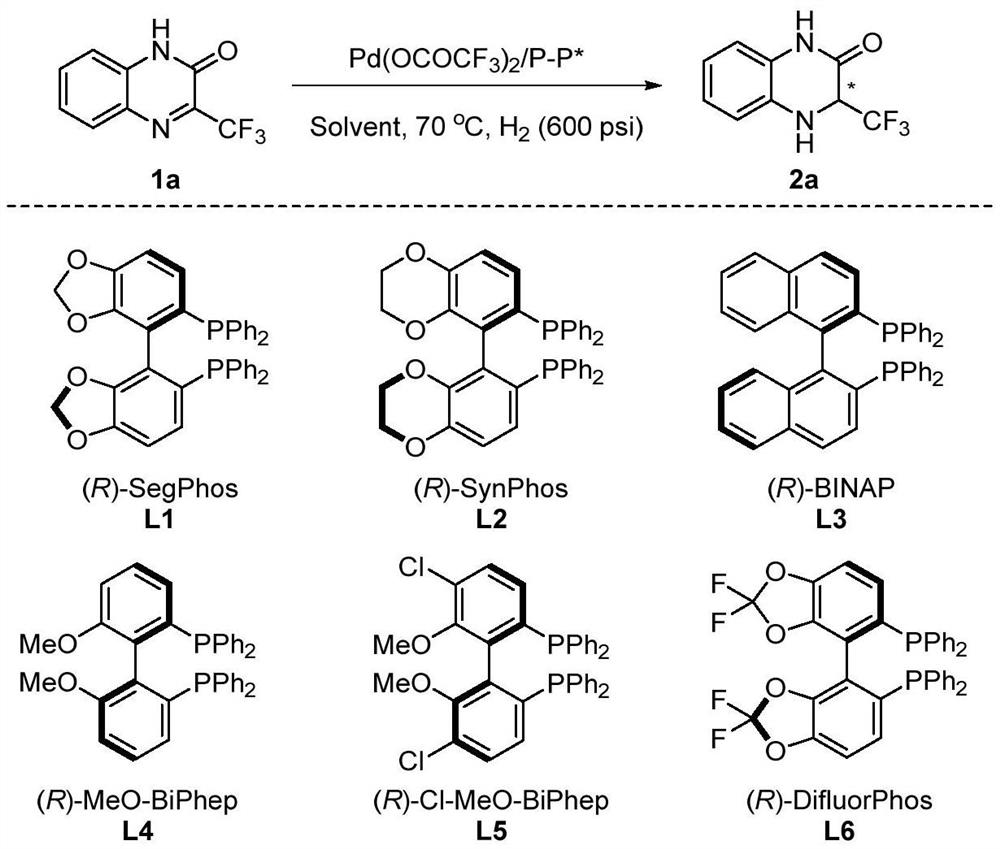
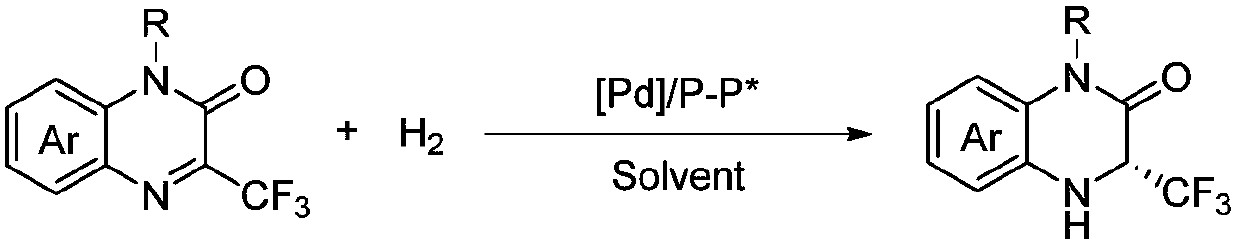
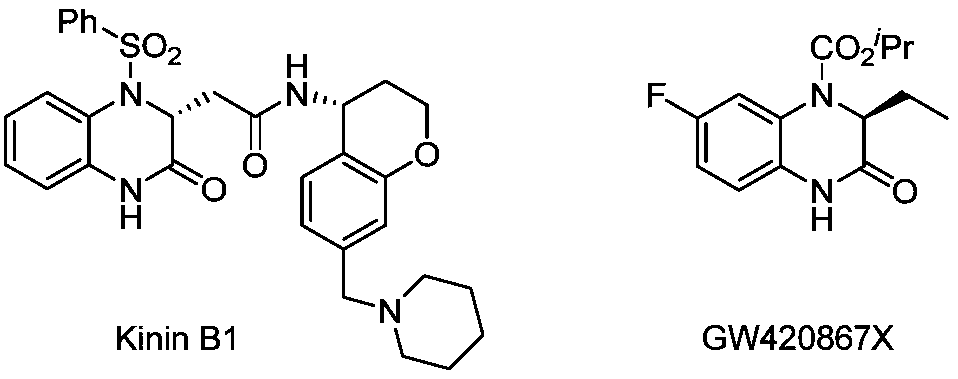
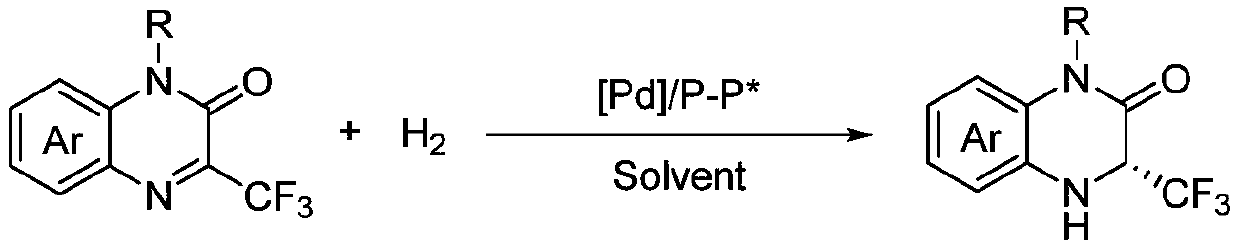
Method for synthesizing chiral 3-trifluoromethyl-3, 4-dihydroquinoxalinone by palladium-catalyzed asymmetric hydrogenation
The invention relates to a method for synthesizing chiral 3-trifluoromethyl-3, 4-dihydroquinoxalinone by palladium-catalyzed asymmetric hydrogenation. A catalytic system is a chiral diphosphorus complex of palladium, and the reaction conditions are as follows: the temperature is 0-80 DEG C, the solvent is 2, 2, 2-trifluoroethanol or hexafluoroisopropanol, the pressure ranges from 100 psi to 1000 psi, the ratio of the substrate to the catalyst is 33 / 1, the used metal precursor is palladium trifluoroacetate, and the used chiral ligand is a chiral diphosphorus ligand. The preparation method of the catalyst comprises the following steps: stirring a metal precursor of palladium and a chiral diphosphine ligand in acetone at room temperature, and then carrying out vacuum concentration to obtain the catalyst. Corresponding chiral dihydroquinoxalinone containing trifluoromethyl can be obtained by hydrogenating quinoxalinone containing trifluoromethyl, and the enantiomeric excess of the dihydroquinoxalinone containing trifluoromethyl can reach 99%. The method is simple, convenient and practical to operate, high in enantioselectivity and high in yield, and the reaction has green atom economyand is environmentally friendly.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
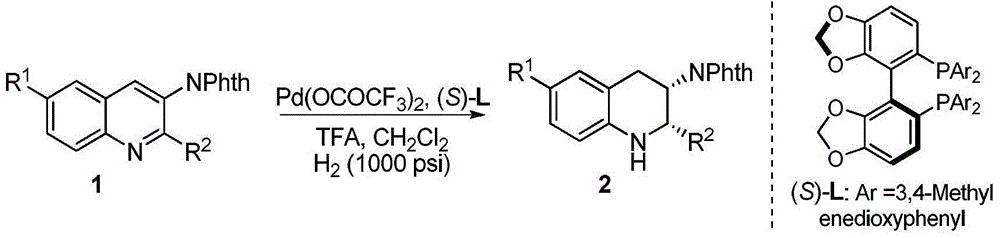
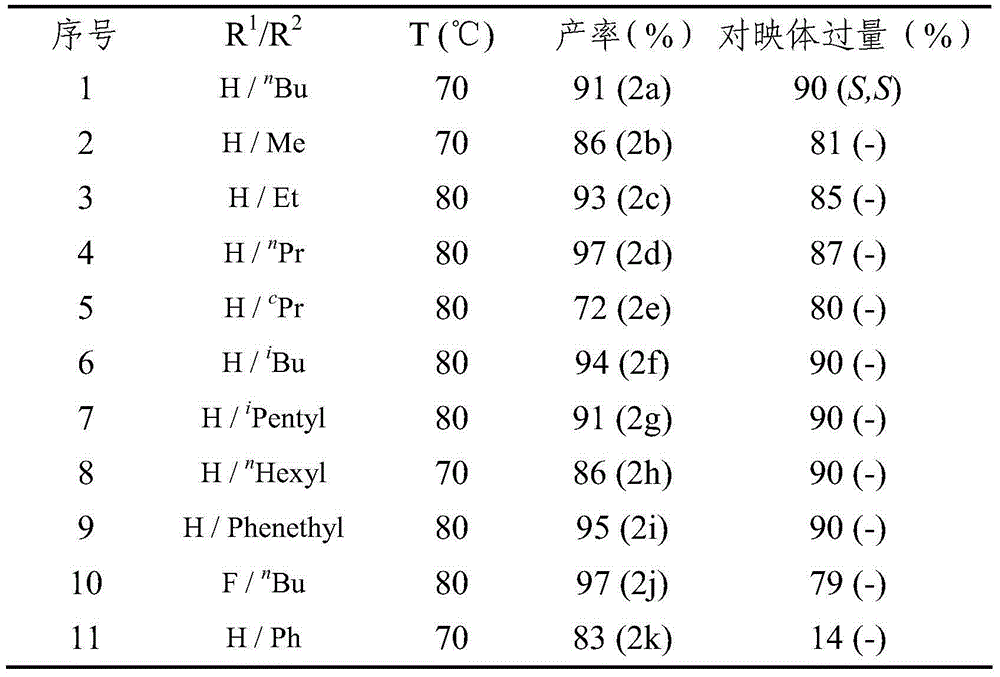
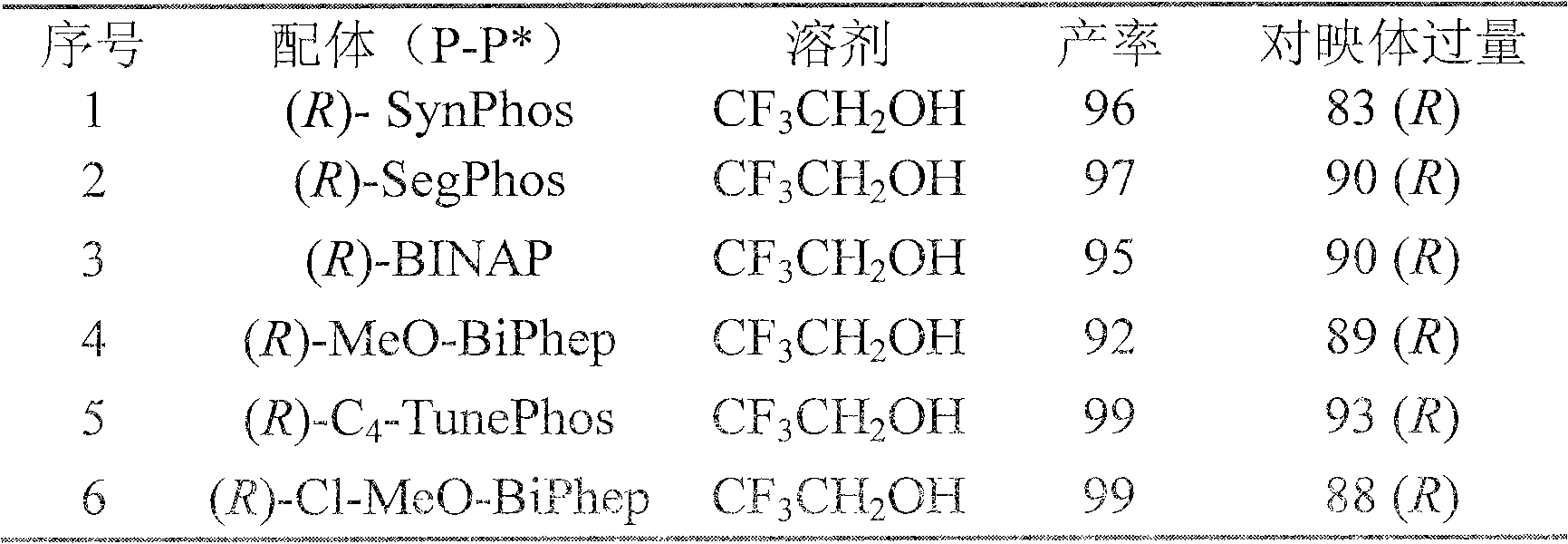
Method for synthesizing chiral amine by asymmetric hydrogenation of iridium-catalyzed pyrrole/indol [1,2-a] quinoxaline
ActiveCN109810109AHigh reactivityHigh enantioselectivityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsIridiumQuinoxaline
The invention provides a method for synthesizing chiral amine. Catalyzed pyrrole / indol [1,2-a] quinoxaline is prepared through asymmetric hydrogenation reaction, a catalyst is the complex of a metal precursor and chiral bisphosphine ligand of iridium, reaction activity and enantioselectivity are high, two kinds of hydrogenation products, i.e., an in situ protected hydrogenation product and a direct hydrogenation product, can be respectively or simultaneously obtained through regulating and controlling the added quantity and reaction time of acetic anhydride, a corresponding chiral tertiary amine compound is obtained, one or two kinds of high enantiomeric excess pure products can be obtained, and the enantiomeric excess of the pure products can be up to 97% at most. The method is simple, convenient, practical and easy in operation, high in yield and friendly to the environment, the catalyst can be commercially obtained, the reaction conditions are mild, and therefore, the method has a potential practical application value.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
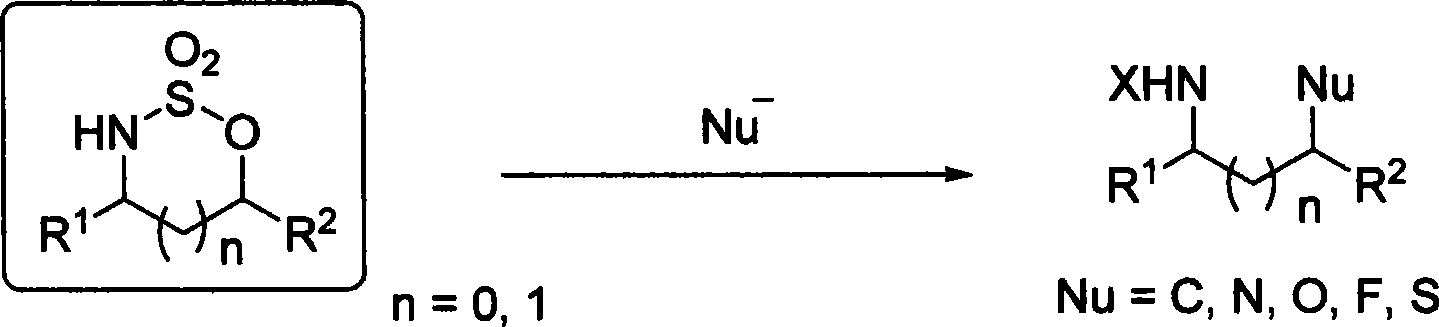
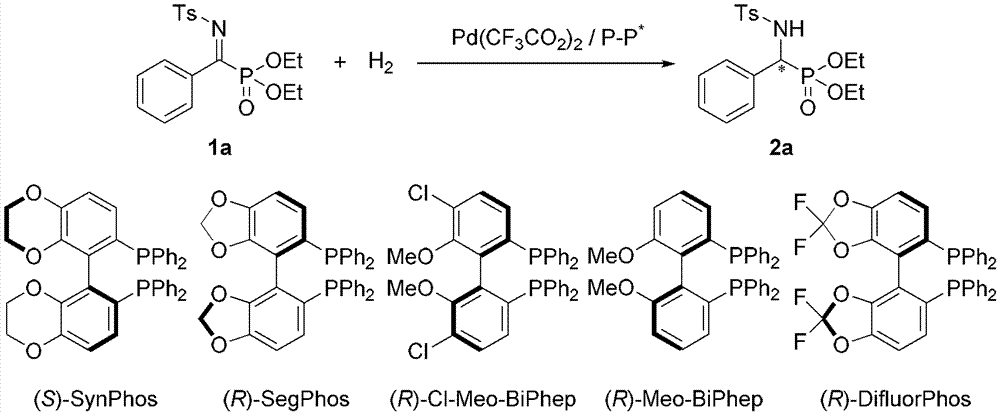
Method for synthesizing chiral amine through palladium catalyzed asymmetric hydrogenolysis of racemic oxazirine
A catalysis system used in a method for synthesizing chiral amine through palladium catalyzed asymmetric hydrogenolysis of racemic oxazirine is a chiral diphosphine complex of palladium. The racemic substituted oxazirine is hydrogenolyzed to obtain corresponding chiral sulfonamide, and the enantiomeric excess can reach 99%. The method has the advantages of simple, practical and easy operation, commercially available catalyst and mild reaction conditions. The method for synthesizing the chiral sulfonamide through asymmetric hydrogenolysis also has the advantages of high antipodal selectivity, good yield, environmentally-friendly and atomically-economic reaction, and environmental protection.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
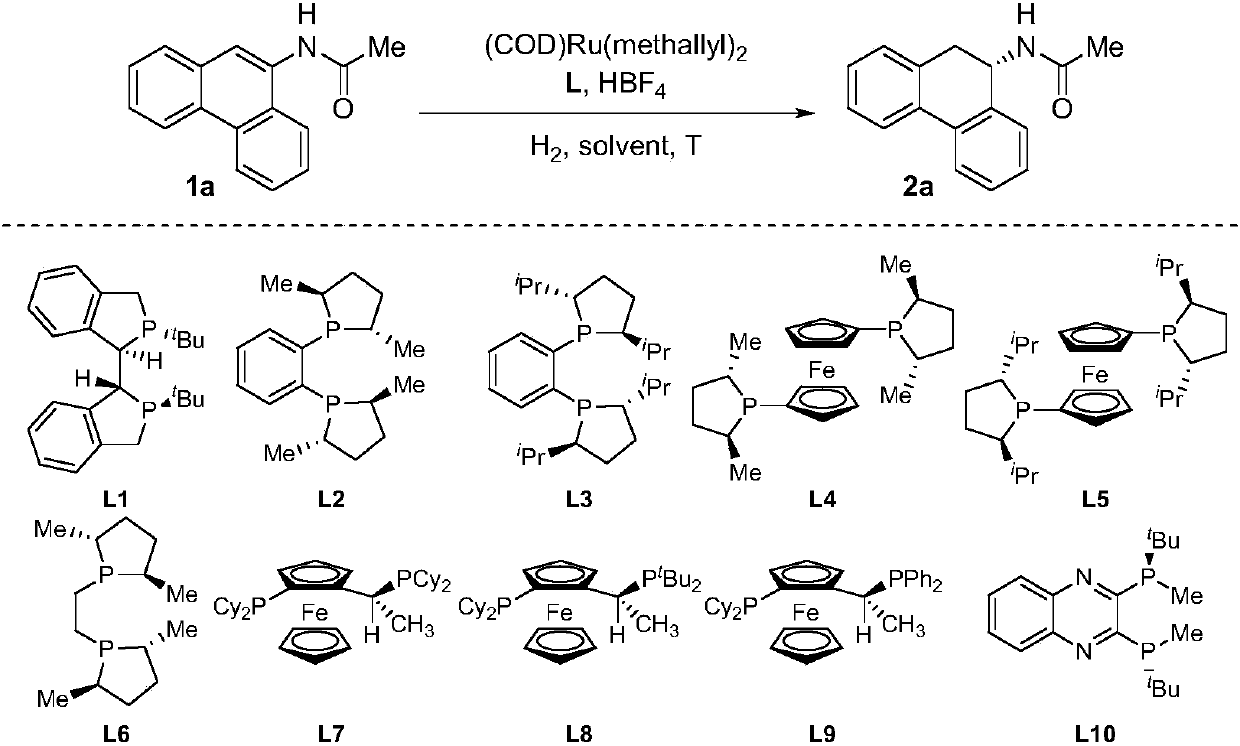
Method for synthesizing chiral tertiary amine by asymmetric hydrogenation of arylamine compound under catalysis of ruthenium
ActiveCN109574867AHigh reactivityHigh enantioselectivityOrganic compound preparationGroup 5/15 element organic compoundsAsymmetric hydrogenationDiphosphines
The invention discloses a method for synthesizing chiral tertiary amine by asymmetric hydrogenation of a 9-amide phenanthrene compound under catalysis of a ruthenium-diphosphine ligand. The method includes: adopting 2-5mol% of a ruthenium catalyst, and adding 4-10mol% of fluoboric acid for asymmetric hydrogenation of the 9-amide phenanthrene compound to obtain a corresponding chiral tertiary aminecompound, wherein the enantiomeric excess reaches 98%. The method is simple, convenient, practical and feasible in operation, high in yield, environmentally friendly and mild in reaction condition, the catalyst is commercially acquirable, and a potential practical application value is achieved.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
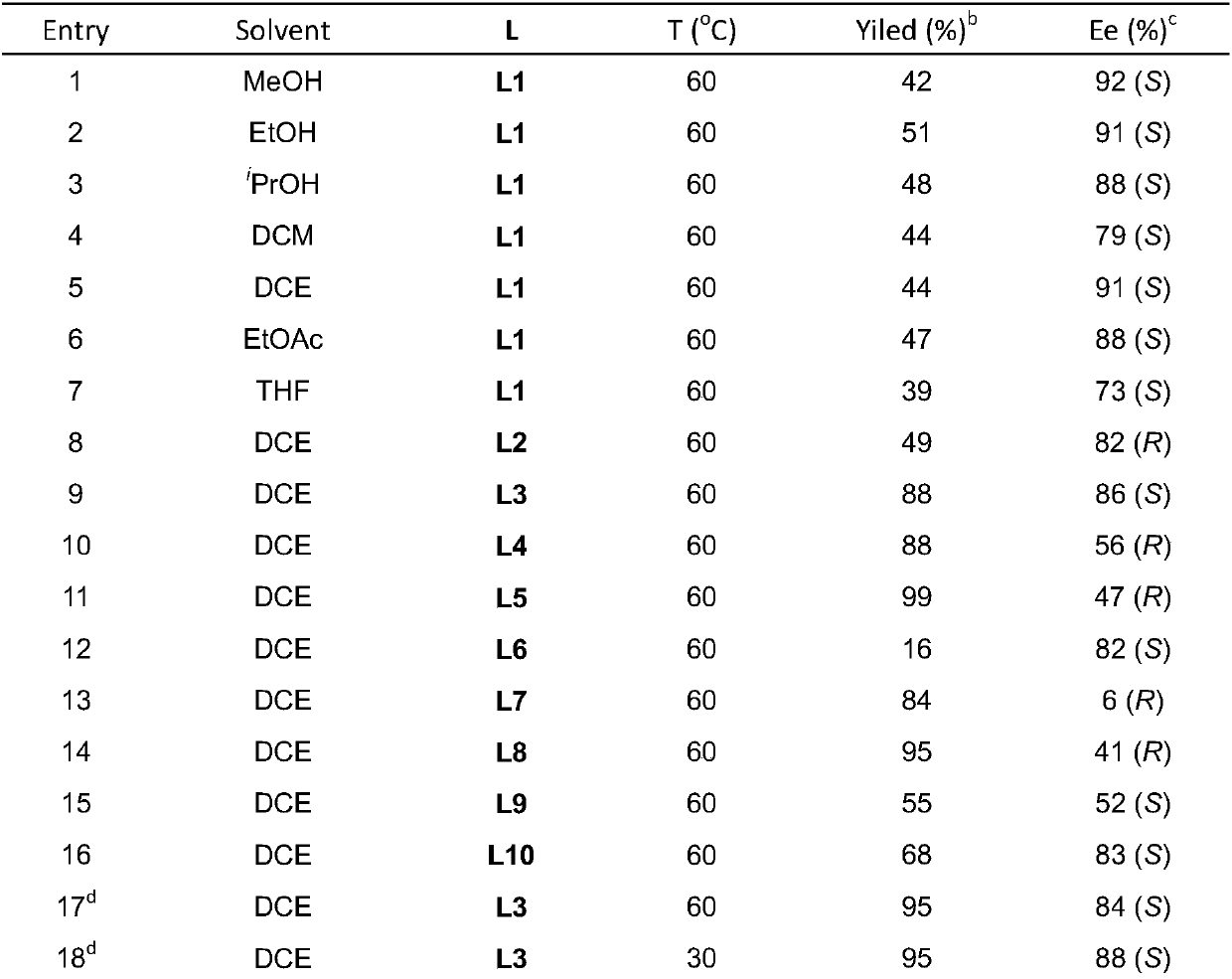
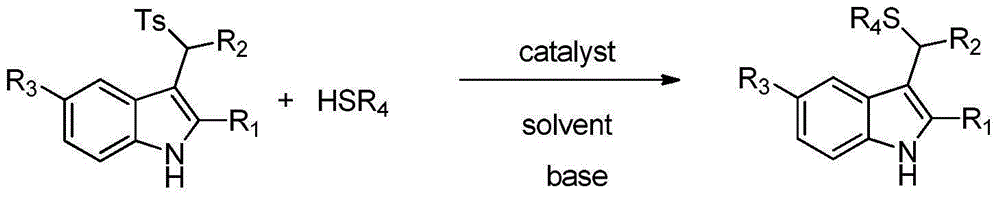
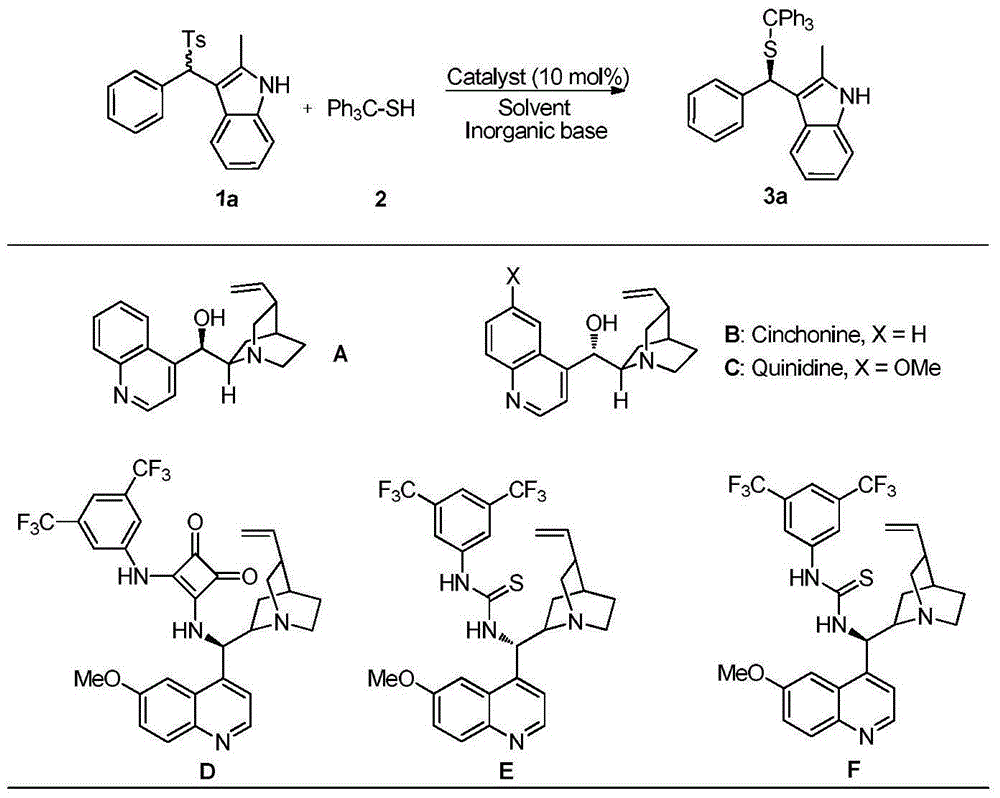
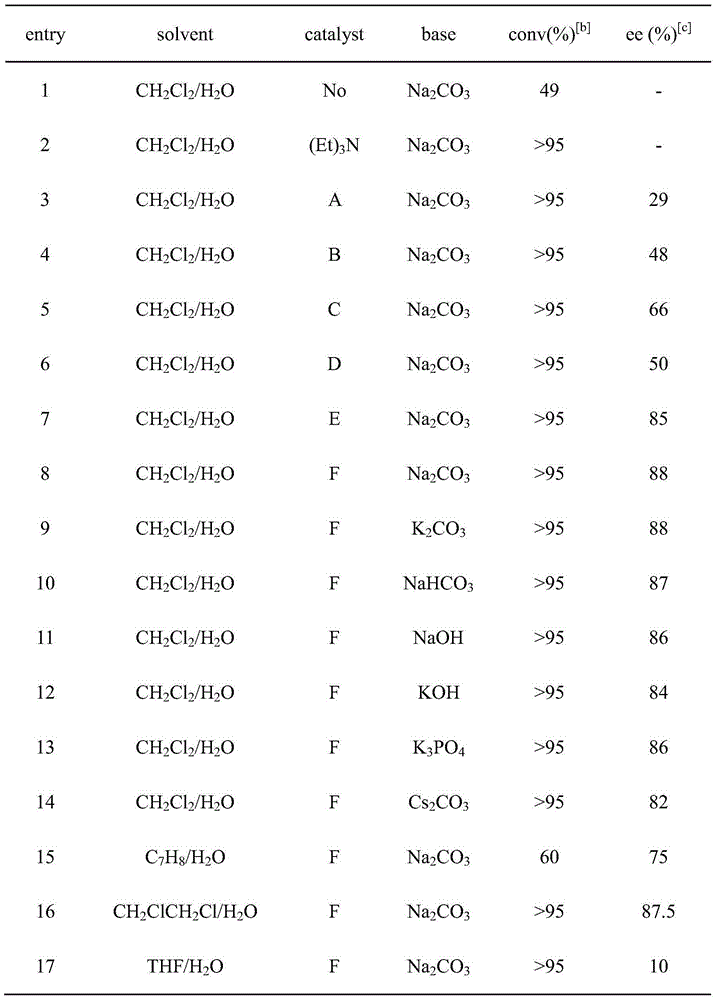
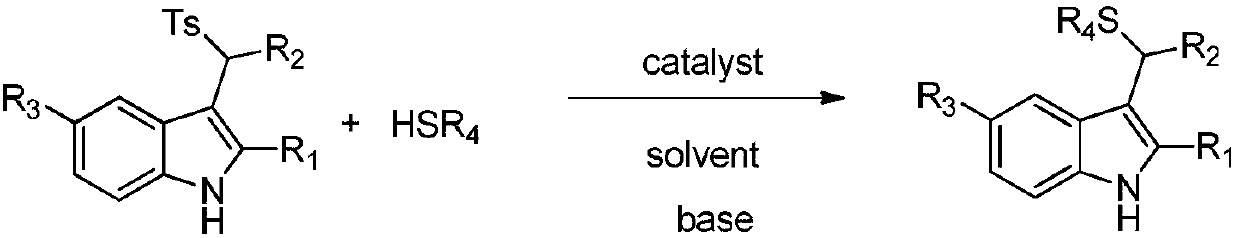
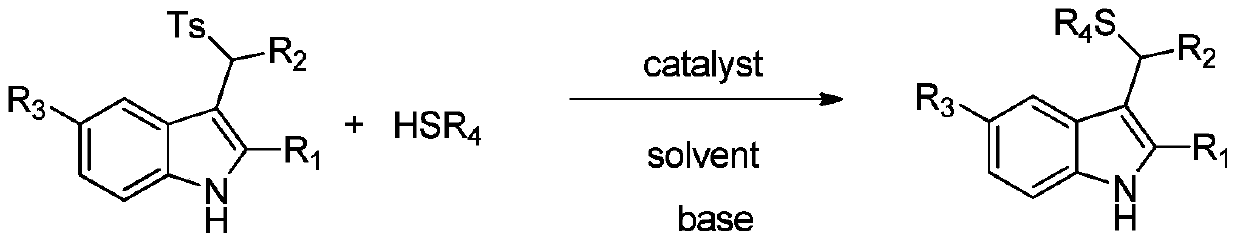
Applications of chiral organic alkali in preparation of chiral sulfur-containing indole compound in water-oil two-phase system
ActiveCN106366032AHigh reactivityHigh enantioselectivityOrganic-compounds/hydrides/coordination-complexes catalystsAsymmetric synthesesThiolOrganic base
The invention provides applications of a chiral organic alkali in preparation of a chiral sulfur-containing indole compound in a water-oil two-phase system, particularly a method for asymmetrically vulcanizing indole by catalyzing mercaptan in a water-oil two-phase system through chiral organic alkali small molecule, wherein the used catalyst is a small molecule chiral organic alkali, the vinylogous imine intermediate generated from racemic p-toluenesulfonyl indole under an alkali condition is vulcanized so as to obtain the corresponding chiral indole sulfide, the yield can achieve 99%, and the enantiomer excess can achieve 98%. According to the present invention, the operation is simple and practical, and is easy to operate, the catalyst is commercially available, the water is used as the solvent, the reaction conditions are mild and green, the yield is high, the enantioselectivity is good, and the reaction is performed in the water phase so as to provide the advantage of environmental protection; and the chiral indole sulfide synthesized through the asymmetric vulcanizing and the indoline sulfide obtained through the derivatization have potential medical values.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for synthesizing chiral dihydro-6H-benzpyrole-[2,1-c][1,4]-benzodiazepine-6-ketone
InactiveCN103288831AHigh reactivityHigh enantioselectivityAsymmetric synthesesBenzodiazepineEnantiomer
The invention discloses a method for synthesizing chiral dihydro-6H-benzpyrole-[2,1-c][1,4]-benzodiazepine-6-ketone by asymmetric hydrogenation under catalysis of iridium. The reaction conditions are that the temperature is 0-50 DEG C; a solvent adopts a dichloromethane and methylbenzene mixed solvent in which the volume ratio of dichloromethane to methylbenzene is 1 to 2; the pressure is 13-50 atm; the proportion of a primer to a catalyst is 50 to 1; the catalyst is a complex consisting of (1,5-cyclooctadiene) iridium chloride dimer and diphosphine; an additive is morpholine trifluoroacetate or piperidine hydrochloride; a corresponding chiral dihydro product is obtained by hydrogenating seven-membered ring-shaped benzpyrole-[2,1-c][1,4]-benzodiazepine-6-ketone; enantiomer is excessive and can reach 96 percent. The method is easy to operate and practicable; raw materials are readily available; the enantioselectivity is high; the yield is high.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
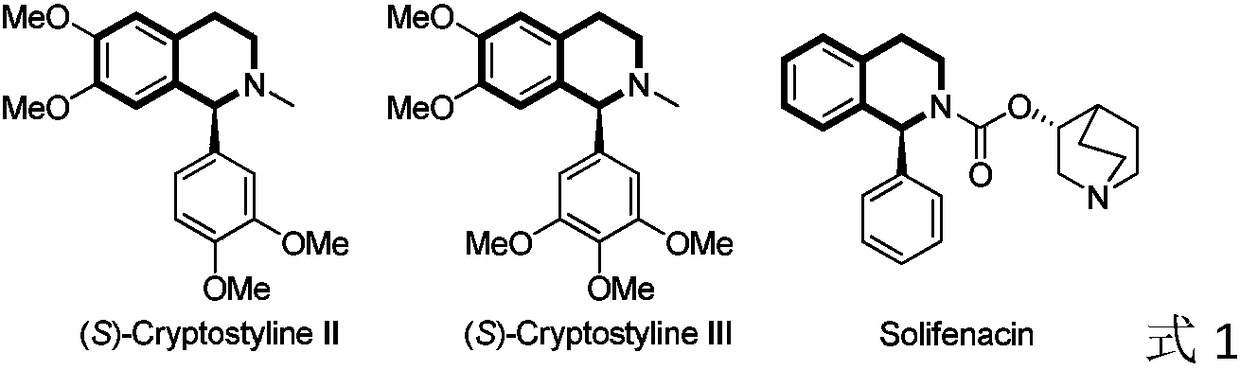
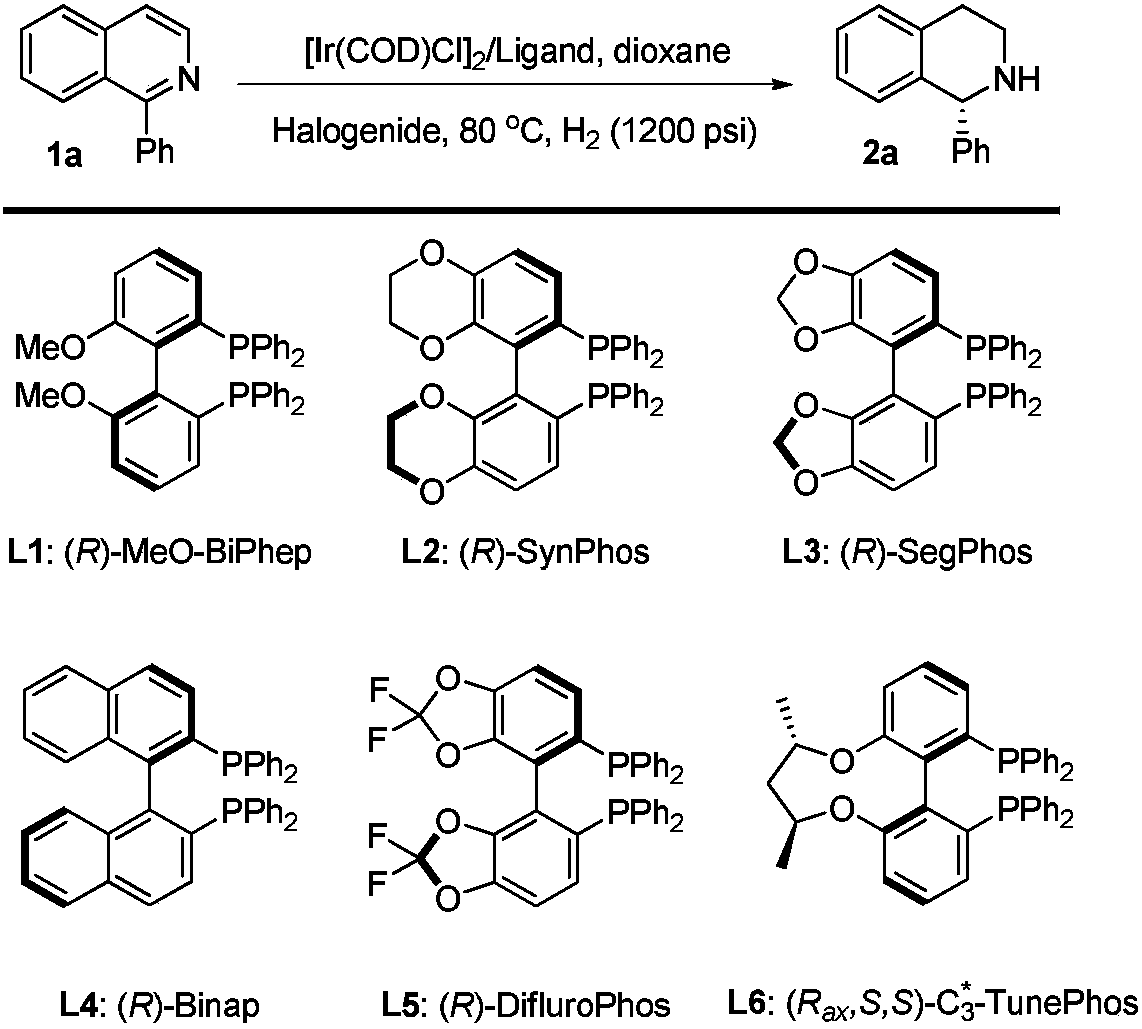
Method for asymmetric hydrogenation of isoquinoline by activation of halogen bond
InactiveCN109422682AHigh reactivityHigh enantioselectivityOrganic chemistry methodsOrganic-compounds/hydrides/coordination-complexes catalystsDiphosphinesBiological activation
Disclosed is a method for asymmetric hydrogenation of isoquinoline by activation of a halogen bond. A catalytic system used by the method is a chiral diphosphine complex of iridium, and an activator is halides. An reaction can be carried out under the following conditions: the temperature is 25-100 DEG C, a solvent is tetrahydrofuran, the pressure is 13-100 atmospheres, a ratio of a substrate to acatalyst is 50 / 1, and the catalyst is a complex of (1,5-cyclooctadiene) iridium chloride dimer and a bisphosphine ligand. The isoquinoline is hydrogenated to obtain corresponding chiral tetrahydroisoquinoline derivatives, and an enantiometric excess can reach 99%. The method has the advantages of simple and practical operation, easy availability of raw materials, high enantioselectivity and goodyield, and the reaction has the advantages of green atom economy, environmental friendliness and the like.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for synthesizing chiral fluoroamine by palladium catalytic asymmetric hydrogenation
ActiveCN102336621BHigh reactivityHigh enantioselectivityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsSolventFluoroamine
The invention discloses a method for synthesizing chiral fluoroamine by palladium catalytic asymmetric hydrogenation, wherein, the used catalysis system comprises a palladium chiral diphosphite complex. The reaction is carried out under the following conditions that: the temperature is 0-50 DEG C, the solvent is a 2,2,2-trifluoroethyl alcohol, the pressure is 1-42 atm, the ratio of the substrate to the catalyst is 50 : 1, the used metal precursor is palladium trifluoroacetate, the used chiral ligand is a chiral diphosphite ligand. The catalyst is prepared by stirring the palladium metal precursor and the chiral diphosphite ligand in acetone at room temperature, and then carrying out vacuum condensation. According to the invention, by the hydrogenation of imine containing trifluoromethyl, corresponding chiral amine containing trifluoromethyl is obtained, the enantiomeric excess can reach to 94 %; by the hydrogenation of imine containing perfluoroalkyl, corresponding chiral amine containing perfluoroalkyl is obtained, and the enantiomeric excess can reach to 86 %. The present invention has the advantages of simple and practical operation, high enantioselectivitiy, good yield, green atom economy of the reaction, and no environmental pollution.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
A method for iridium-catalyzed asymmetric hydrogenation of quinoline-3-amine to synthesize chiral exocyclic amine
ActiveCN104710406BEasy to separateHigh reactivityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsAsymmetric hydrogenationDiphosphines
A method for synthesizing chiral exocyclic amine by iridium-catalyzed asymmetric hydrogenation of quinoline-3-amine, the catalytic system used is chiral diphosphorus complex of iridium. The reaction can be carried out under the following conditions, temperature: 25-70°C; solvent: a mixed solvent of toluene / tetrahydrofuran (V / V=3:1); pressure: 2-14 atmospheres; the ratio of substrate to catalyst is 25 / l; The catalyst is a complex of (1,5-cyclooctadiene) iridium chloride dimer and a chiral bisphosphine ligand. For quinoline-3-amine, the corresponding chiral exocyclic amine derivative can be obtained, and its enantiomeric excess value can reach 94%. The invention has the advantages of simple and practical operation, readily available raw materials, high enantioselectivity and good yield, and the reaction has green atom economy and is environmentally friendly.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
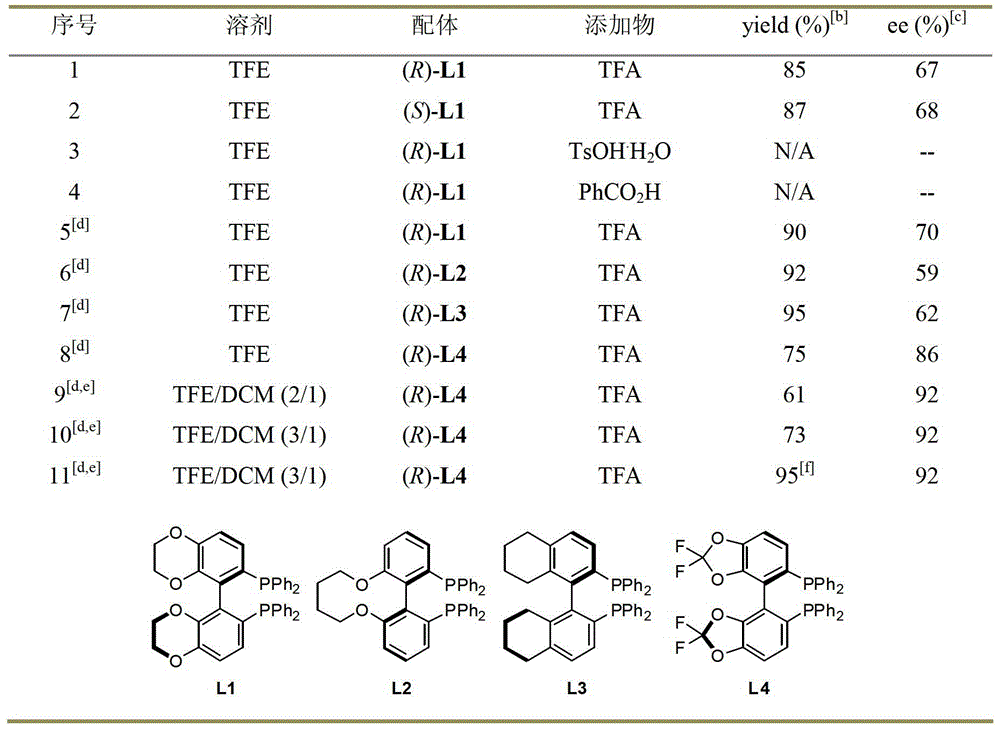
A kind of method for synthesizing chiral amine by asymmetric hydrogenolysis of palladium-catalyzed amino alcohol
ActiveCN104418775BHigh reactivityHigh enantioselectivityOrganic compound preparationSulfonic acid amide preparationEnantiomerDiphosphines
A method for palladium-catalyzed asymmetric hydrogenolysis of N-sulfonyl amino alcohol compounds, the reaction conditions are as follows, temperature: 25-70 degrees; solvent: 2,2,2-trifluoroethanol / dichloromethane; pressure: 28-40 Atmospheric pressure; The ratio of substrate and catalyst is 50 / l; The catalyst is the complex of palladium trifluoroacetate and bisphosphorus ligand; The hydrogenolysis of the five-membered ring N-sulfonyl amino alcohol of p-benzo obtains the corresponding chiral The enantiomeric excess of benzopenta-membered N-sulfonyl amino compounds can reach 94%. The invention has the advantages of simple and practical operation, high enantioselectivity and good yield, and the reaction has green atom economy and is friendly to the environment.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
A method of a catalytic asymmetric molecule internal amine synthetic sulfa
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Application of chiral organic bases in preparation of chiral sulfur-containing indole compounds in water-oil two-phase system
ActiveCN106366032BGreen and mild reaction conditionsHigh reactivityOrganic-compounds/hydrides/coordination-complexes catalystsAsymmetric synthesesThiolSulfide
The application of chiral organic bases in the preparation of chiral sulfur-containing indole compounds in the water-oil two-phase system is a small molecule of chiral organic bases in the water-oil two-phase system to catalyze the asymmetric vulcanization of mercaptans to indole, The catalyst used is a small molecule chiral organic base. The corresponding chiral indole sulfide can be obtained after sulfiding the vinylene imine intermediate generated by the racemic p-toluenesulfonyl indole under alkaline conditions, and the yield can reach 99%. The enantiomeric excess can reach 98%. The invention is simple, practical and easy to operate, the catalyst is commercially available, water is used as the solvent, the reaction condition is green and mild, the yield is high, the enantioselectivity is good, and the reaction is carried out in the water phase, which is environmentally friendly. In addition, the synthesis of chiral indole sulfide and derivatized indoline sulfide through asymmetric sulfidation have potential medical value.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for synthesizing chiral benzosultam via palladium-catalytic asymmetric hydrogenation
The invention discloses a method for synthesizing chiral benzosultam via palladium-catalytic asymmetric hydrogenation, comprising the following reaction conditions that: the temperature is 25-70DEG C; a solvent is 2,2,2-trifluoroethanol; the pressure is 28-40atm; the proportion of a substrate to a catalyst is 50 / 1; the catalyst is a complex of palladium trifluoroacetate and diphosphine ligand; the benzo six-membered ring shaped sulfonyl enamine of the benzo is hydrogenated to obtain the corresponding chiral benzo six-membered sultam with an enantiomeric excess up to 98%; and the benzo five-membered ring shaped sulfonyl enamine is hydrogenated to obtain the corresponding chiral benzo five-membered sultam with an enantiomeric excess up to 94%. The method disclosed by the invention is simple, convenient and practical for operation, high in enantioselectivity, good in yield and environmentally friendly, and has green atom economy for the reaction.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Catalytic asymmetric hydrogenation synthesis method for chiral gamma-sultam
InactiveCN101260085BHigh reactivityHigh enantioselectivityOrganic chemistrySynthesis methodsDiphosphines
The invention discloses a method using palladium to catalyze asymmetrical hydrogen to compound into chiral gamma-sultam, wherein, a catalytic system used by the invention is a chiral diphosphine complex of palladium. The reaction can be made within the following conditions: temperature: 25 to 75 DEG C; solvent: 2, 2, 2-trifluoroethanol; pressure: 35 to 40 atmospheric pressures; time: 10 to 12 hours. The proportion of substrate and catalyst is 50:1, a metal precursor of the catalyst is palladium trifluoroacetate; a chiral ligand is a chiral diphosphine ligand; the preparation method for the catalyst is that: the metal precursor of the palladium and the chiral diphosphine ligand are stirred in acetone at room temperature, and then are made a vacuum condensation to obtain the catalyst. To hydrogenise cyclic sulfimide can obtain corresponding chiral 3-substituted gamma-sultam, and enantiomeric excess thereof can reach 93 percent. The method has simple and practical operation, easy-obtaining raw materials, high enantioselectivity and good yield; the reaction has green atomic economy and is environment-friendly.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
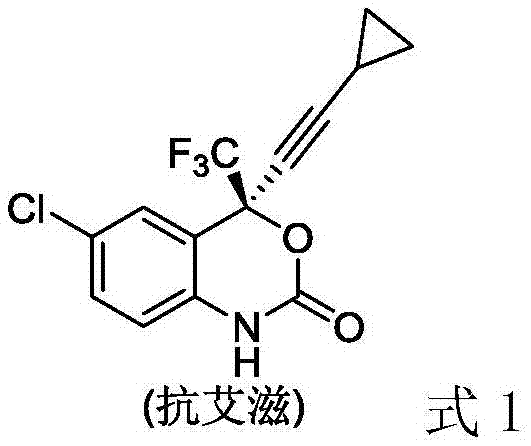
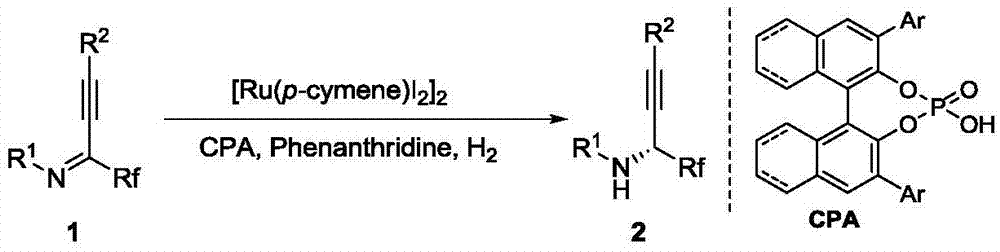
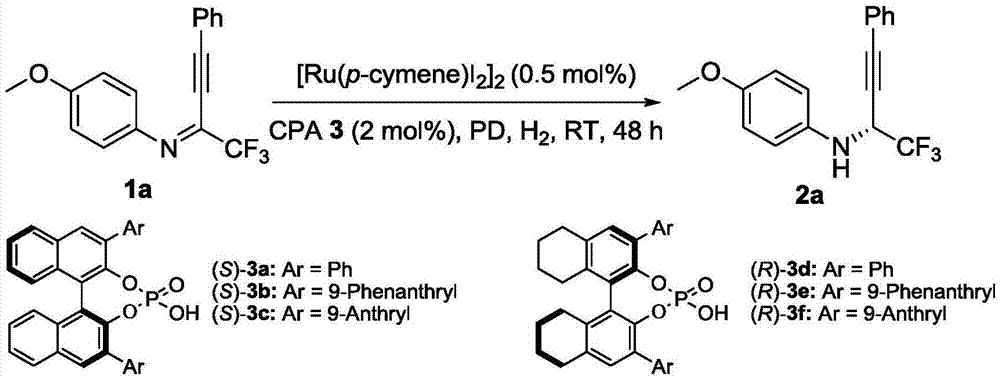
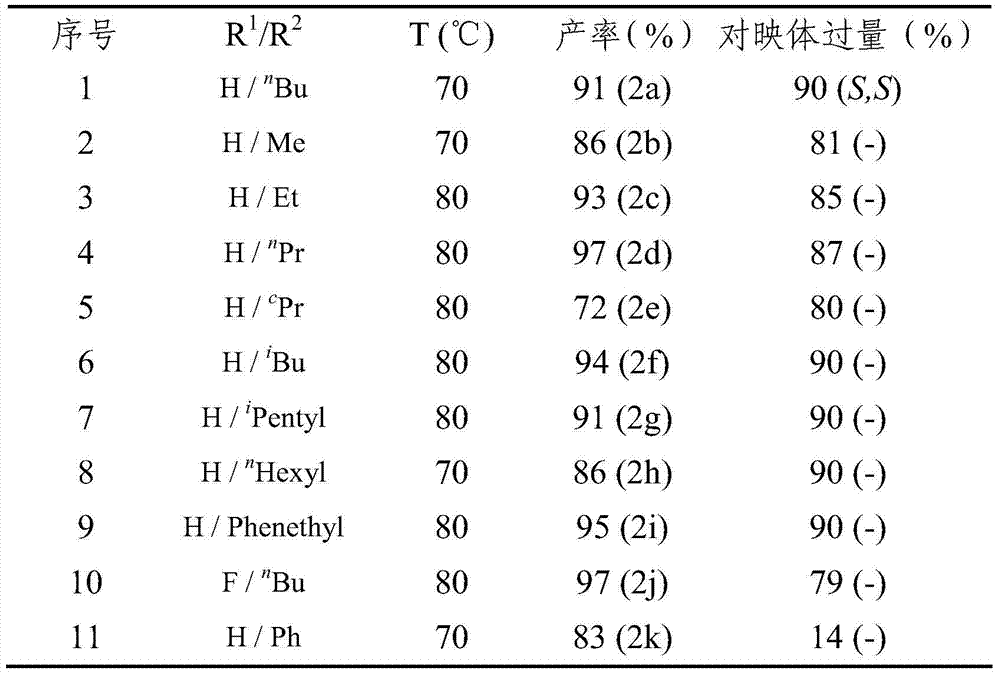
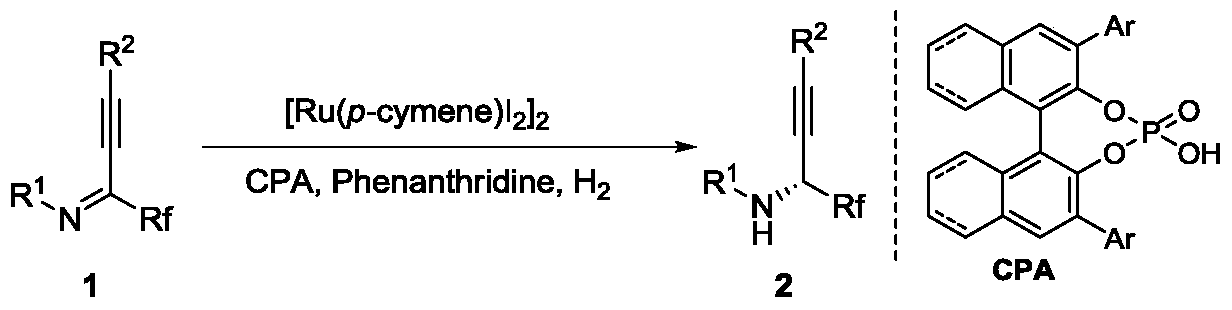
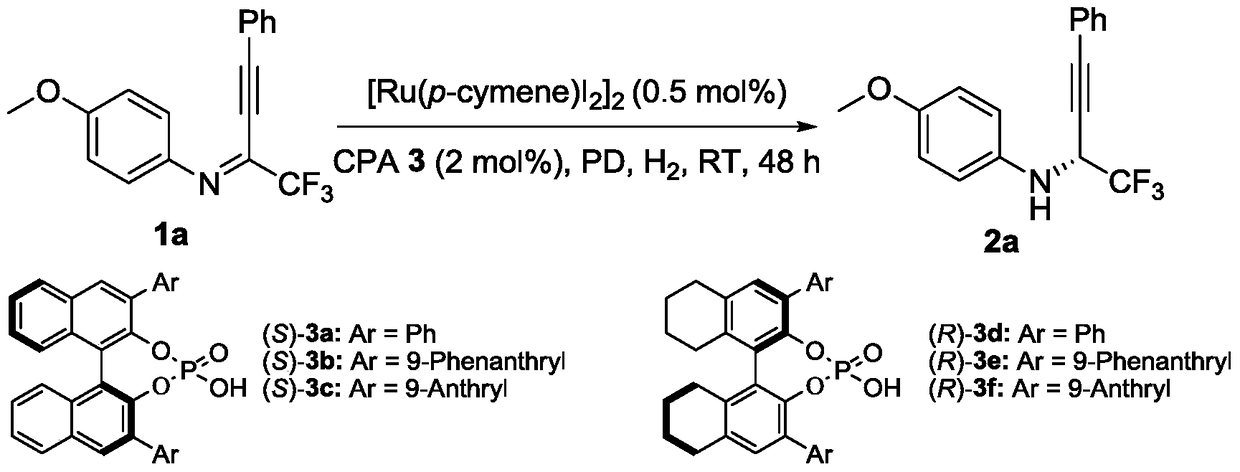
Method for synthesizing chiral fluorine-containing propargylamine derivative by using biomimetic catalysis asymmetric hydrogenation
ActiveCN106995377AHigh reactivityHigh chemoselectivityOrganic compound preparationOrganic chemistry methodsIodidePhosphoric acid
The invention relates to a method for synthesizing a chiral fluorine-containing propargylamine derivative by using biomimetic catalysis asymmetric hydrogenation. A used catalyzing system is ruthenium and chiral phosphoric acid. Reaction can be performed under the following conditions that the temperature is 25 to 100 DEG C; a solvent is 1,2-dichloroethane; the pressure is 13 to 80 atmospheric pressure; the ratio of a primer to a catalyst is 100 / 1; the catalyst is (4-cymene)ruthenium iodide dimer and the chiral phosphoric acid. By using the method, the corresponding chiral fluorine-containing propylamine derivative is obtained by selective hydrogenation using fluorine-containing alkynyl to substitute imine, and the enantiomeric excess can reach 98%. The method has the advantages that the operation is simple, convenient and practical, the obtaining of raw materials is easy, the enantioselectivity is good, and the yield rate is good; the reaction has green atom economy, the environment-friendly effect is realized, and the like.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
A method for the synthesis of chiral tertiary amines by asymmetric hydrogenation of ruthenium catalyzed arylamine compounds
ActiveCN109574867BHigh reactivityHigh enantioselectivityOrganic compound preparationGroup 5/15 element organic compoundsPtru catalystBoronic acid
The invention discloses a method for synthesizing chiral tertiary amine by asymmetric hydrogenation of 9-amidophenanthrene compound catalyzed by ruthenium-bisphosphine ligand. Using 2-5mol% ruthenium catalyst, adding 4-10mol% fluoboric acid, asymmetric hydrogenation of 9-amide phenanthrene compound to obtain the corresponding chiral tertiary amine compound, and its enantiomeric excess can reach 98%. The invention has the advantages of simple and practical operation, high yield, environmental friendliness, commercially available catalyst, mild reaction conditions and potential practical application value.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Nitrogen-doped rhodium carbon catalyst as well as preparation method and application thereof
ActiveCN113499794AHigh reactivityHigh regional selectivityOrganic compound preparationCatalyst activation/preparationActivated carbonPtru catalyst
Owner:SUZHOU SINOCOMPOUND TECH
A kind of palladium-catalyzed quinoline-3-amine asymmetric hydrogenation method for the synthesis of chiral exocyclic amines
ActiveCN105017150BEasy to separateHigh reactivityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsHydrogen pressureQuinoline
A method for palladium-catalyzed asymmetric hydrogenation of quinoline-3-amine to synthesize chiral exocyclic amines is characterized in that Bronsted acid is used as an activator, and the catalytic system used is a chiral bisphosphine complex of palladium. Reaction can be carried out under following conditions, reaction temperature: 60-80 ℃; Reaction adopts solvent: methylene chloride; Hydrogen pressure: 20-70 atmospheric pressure; The molar ratio of substrate and catalyst is 20 / l; Used metal precursor : palladium trifluoroacetate (Pd(OCOCF3)2); chiral ligand used: chiral bisphosphine ligand; activator used: trifluoroacetic acid (CF3CO2H). For quinoline-3-amine, the corresponding chiral exocyclic amine derivative can be obtained, and its enantiomeric excess value can reach 90%. The invention has the advantages of simple and convenient operation, readily available raw materials, good enantioselectivity, high yield, and the reaction has green atom economy and is environmentally friendly.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
A method for the synthesis of chiral fluorine-containing propargylamine derivatives by biomimetic catalytic asymmetric hydrogenation
ActiveCN106995377BHigh reactivityHigh chemoselectivityOrganic compound preparationOrganic chemistry methodsIodidePhosphoric acid
The method for synthesizing chiral fluorine-containing propargylamine derivatives by biomimetic catalysis asymmetric hydrogenation uses ruthenium and chiral phosphoric acid as the catalytic system. Reaction can be carried out in following conditions, temperature: 25-100 ℃; Solvent: 1,2-ethylene dichloride; Pressure: 13-80 atmospheric pressure; The ratio of substrate and catalyst is 100 / l; Catalyst is (4- cymene) ruthenium iodide dimer and chiral phosphoric acid. The selective hydrogenation of fluorine-containing alkynyl-substituted imines affords the corresponding chiral fluorine-containing propylamine derivatives with an enantiomeric excess of up to 98%. The invention has the advantages of simple and practical operation, readily available raw materials, high enantioselectivity, good yield, and the reaction has the advantages of green atom economy and environmental friendliness.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
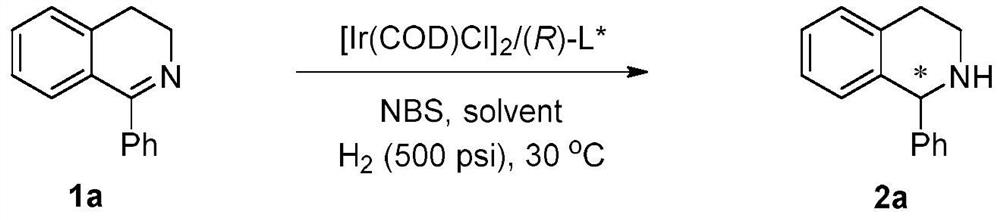
A kind of method of bidirectional enantioselective synthesis of chiral tetrahydroisoquinoline catalyzed by iridium
An iridium-catalyzed two-way enantioselective synthesis method of chiral tetrahydroisoquinoline, the catalyst system used is a chiral bisphosphine complex of iridium. Using a single chiral source, the asymmetric hydrogenation of simple and easy-to-obtain 3,4-dihydroisoquinoline substrates was realized by adjusting the amount of achiral additive N-bromosuccinimide in the reaction system . The method utilizes a single chiral source to obtain two enantiomers of the corresponding chiral tetrahydroisoquinoline respectively, and the enantiomeric excess can reach 89% (S) and 98% (R), respectively. The invention is simple and practical in operation, simple and easy to obtain raw materials, commercially available catalyst, mild reaction conditions, high yield and high enantioselectivity. In addition, the two enantiomers of tetrahydroisoquinoline can be obtained only through the conditions of achiral additives, successfully avoiding the synthesis of two different configuration ligands.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
A method for the synthesis of chiral amines by iridium-catalyzed asymmetric hydrogenation of pyrrole/indolo[1,2-a]quinoxaline
ActiveCN109810109BHigh reactivityHigh enantioselectivityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsQuinoxalineAcetic anhydride
The invention provides a method for synthesizing chiral amine. Catalyzed pyrrole / indol [1,2-a] quinoxaline is prepared through asymmetric hydrogenation reaction, a catalyst is the complex of a metal precursor and chiral bisphosphine ligand of iridium, reaction activity and enantioselectivity are high, two kinds of hydrogenation products, i.e., an in situ protected hydrogenation product and a direct hydrogenation product, can be respectively or simultaneously obtained through regulating and controlling the added quantity and reaction time of acetic anhydride, a corresponding chiral tertiary amine compound is obtained, one or two kinds of high enantiomeric excess pure products can be obtained, and the enantiomeric excess of the pure products can be up to 97% at most. The method is simple, convenient, practical and easy in operation, high in yield and friendly to the environment, the catalyst can be commercially obtained, the reaction conditions are mild, and therefore, the method has a potential practical application value.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
A method for palladium-catalyzed asymmetric hydrogenation of chiral 3-trifluoromethyl-3,4-dihydroquinoxalinone
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
A method for palladium catalyzed asymmetric hydrogenolysis and racemization of oxaziridine to synthesize chiral amines
The invention discloses a palladium-catalyzed asymmetric hydrogenolysis and racemization-substituted oxaziridine method for synthesizing chiral sulfonamides. The catalytic system used in the method is palladium chiral bisphosphine complexes. Hydrogenolysis of racemic substituted oxaziridines can afford the corresponding chiral sulfonamides with an enantiomeric excess of 99%. The invention is simple and practical to operate, the catalyst is commercially available, and the reaction conditions are mild. In addition, the synthesis of chiral sulfonamides by asymmetric hydrogenolysis has high enantioselectivity and good yield, and the reaction is environmentally friendly with green atom economy.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
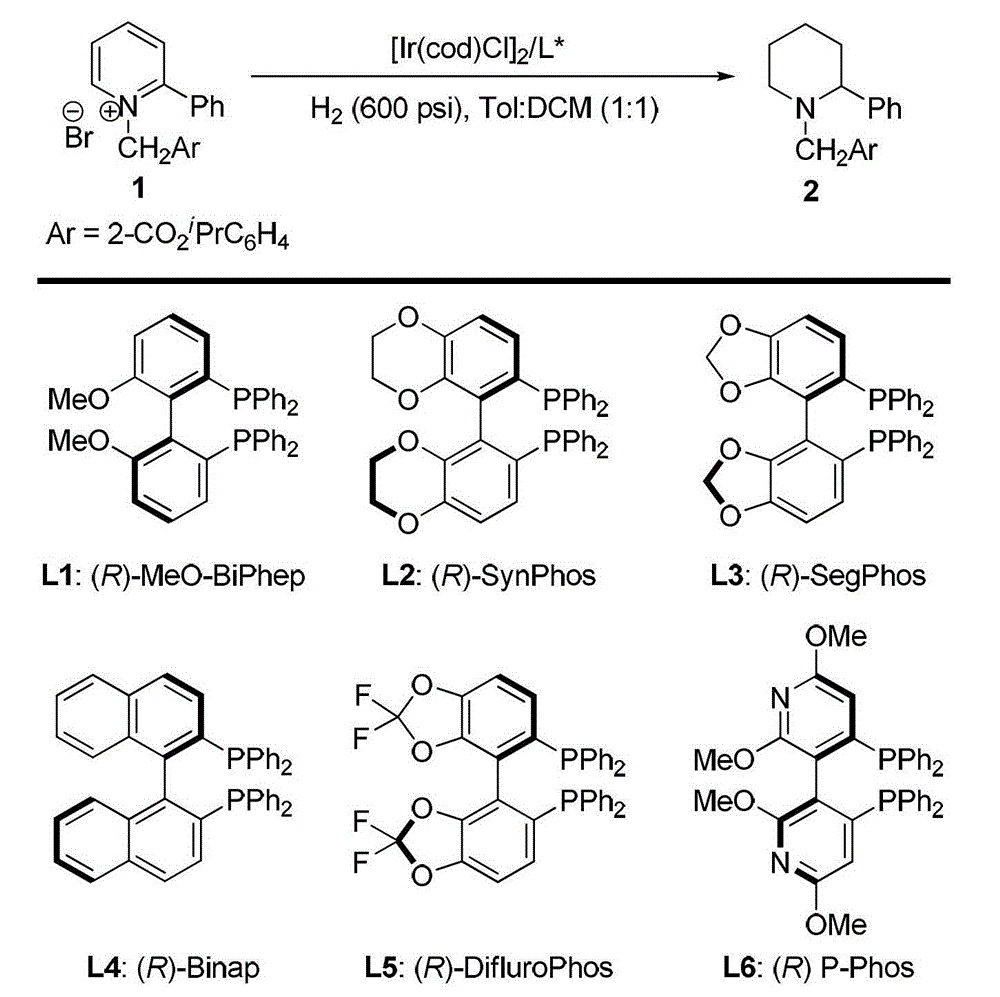
A kind of method for synthesizing chiral piperidine derivatives by asymmetric hydrogenation of pyridine catalyzed by iridium
ActiveCN103387533BHigh reactivityHigh enantioselectivityAsymmetric synthesesDiphosphinesIridium chloride
A method for synthesis of chiral piperidine derivatives through iridium-catalyzed asymmetric hydrogenation of pyridine employs a catalytic system of chiral diphosphine complex of iridium. The reaction can be carried out under the following conditions: temperature of 25-60 DEG C; a mixed solvent of toluene / dichloromethane (V / V=1:1); pressure of 13-50 barometric pressure; a ratio of substrate and catalyst of 50 / 1; and a catalyst of a complex of (1,5-cyclo-octadiene) iridium chloride dimer and diphosphine ligand. Hydrogenation of pyridine salt can obtain a corresponding chiral 2-substituted piperidine derivative, whose enantiomeric excess can reach 93%. The invention has advantages of simple and practical operation, easily available raw materials, high enantioselectivity, and good yield; in addition, the reaction has green atom economy and is friendly to environment.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
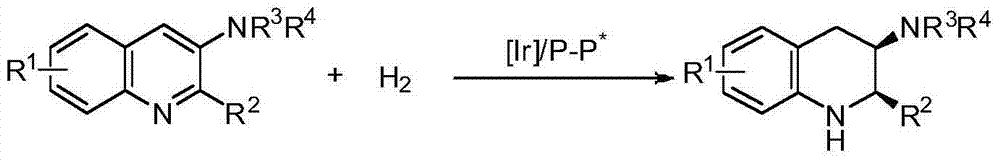
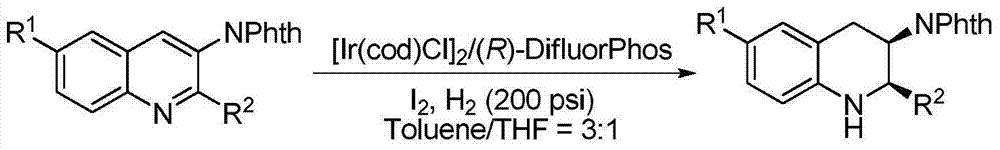
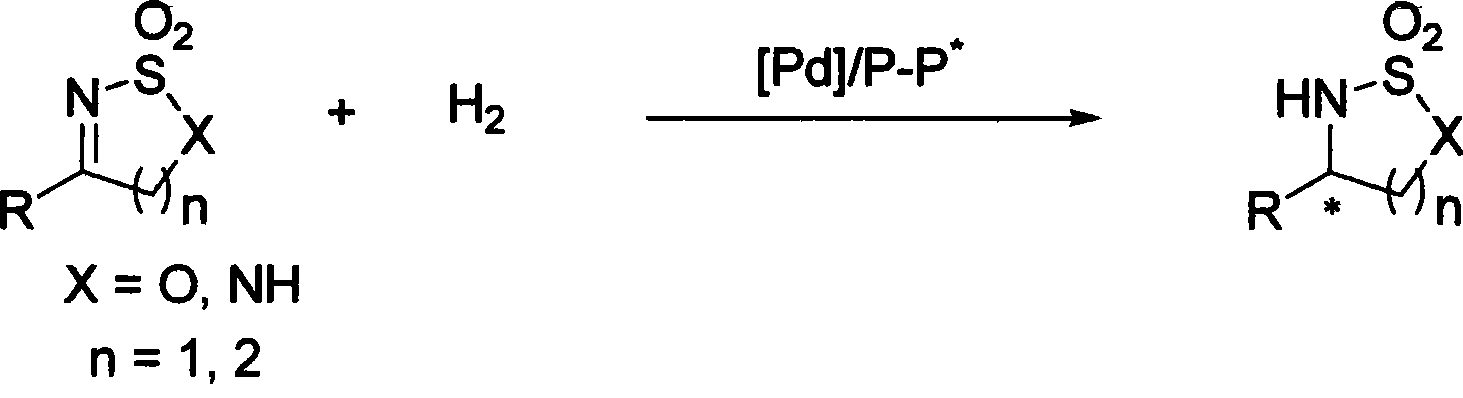
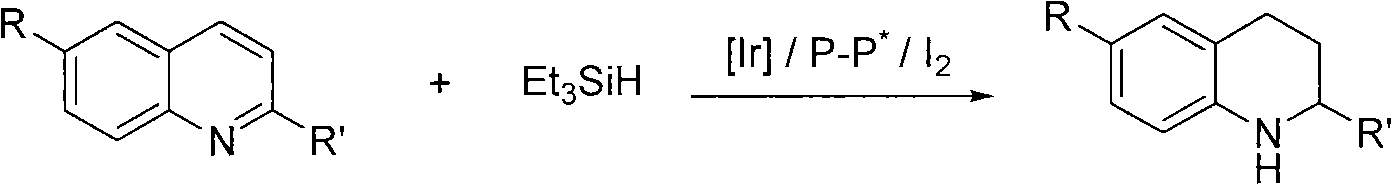
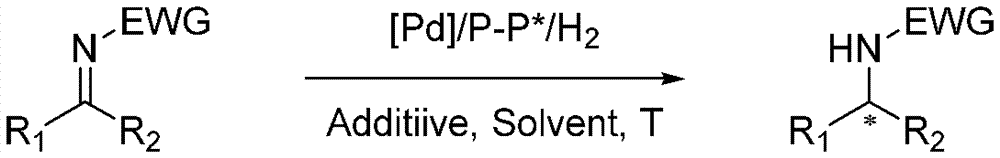
![Method for synthesizing chiral dihydro-5H-pyrrolo[2,1-c][1,4]-benzodiazepino-5-one Method for synthesizing chiral dihydro-5H-pyrrolo[2,1-c][1,4]-benzodiazepino-5-one](https://images-eureka.patsnap.com/patent_img/8721003a-0f8e-4840-b620-3c6dcd1a65ad/FDA0000138253650000011.png)
![Method for synthesizing chiral dihydro-5H-pyrrolo[2,1-c][1,4]-benzodiazepino-5-one Method for synthesizing chiral dihydro-5H-pyrrolo[2,1-c][1,4]-benzodiazepino-5-one](https://images-eureka.patsnap.com/patent_img/8721003a-0f8e-4840-b620-3c6dcd1a65ad/BDA0000138253660000021.png)
![Method for synthesizing chiral dihydro-5H-pyrrolo[2,1-c][1,4]-benzodiazepino-5-one Method for synthesizing chiral dihydro-5H-pyrrolo[2,1-c][1,4]-benzodiazepino-5-one](https://images-eureka.patsnap.com/patent_img/8721003a-0f8e-4840-b620-3c6dcd1a65ad/BDA0000138253660000051.png)
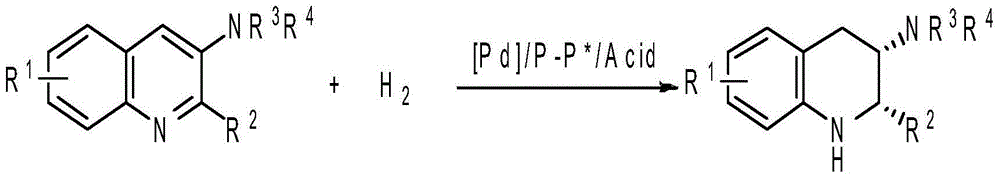
![Method for synthesizing chiral amine by asymmetric hydrogenation of iridium-catalyzed pyrrole/indol [1,2-a] quinoxaline Method for synthesizing chiral amine by asymmetric hydrogenation of iridium-catalyzed pyrrole/indol [1,2-a] quinoxaline](https://images-eureka.patsnap.com/patent_img/5bff97ea-6960-4bfa-a783-ce0822efb6b8/FDA0001474915590000011.png)
![Method for synthesizing chiral amine by asymmetric hydrogenation of iridium-catalyzed pyrrole/indol [1,2-a] quinoxaline Method for synthesizing chiral amine by asymmetric hydrogenation of iridium-catalyzed pyrrole/indol [1,2-a] quinoxaline](https://images-eureka.patsnap.com/patent_img/5bff97ea-6960-4bfa-a783-ce0822efb6b8/FDA0001474915590000012.png)
![Method for synthesizing chiral amine by asymmetric hydrogenation of iridium-catalyzed pyrrole/indol [1,2-a] quinoxaline Method for synthesizing chiral amine by asymmetric hydrogenation of iridium-catalyzed pyrrole/indol [1,2-a] quinoxaline](https://images-eureka.patsnap.com/patent_img/5bff97ea-6960-4bfa-a783-ce0822efb6b8/FDA0001474915590000021.png)
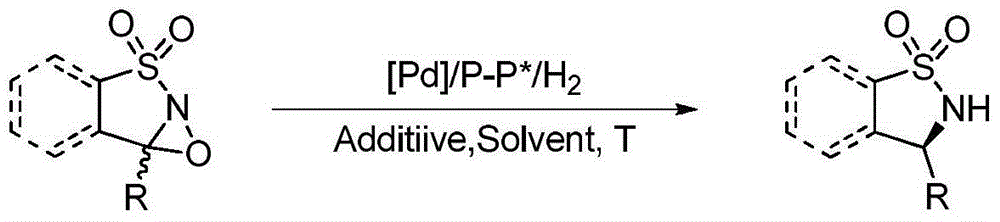
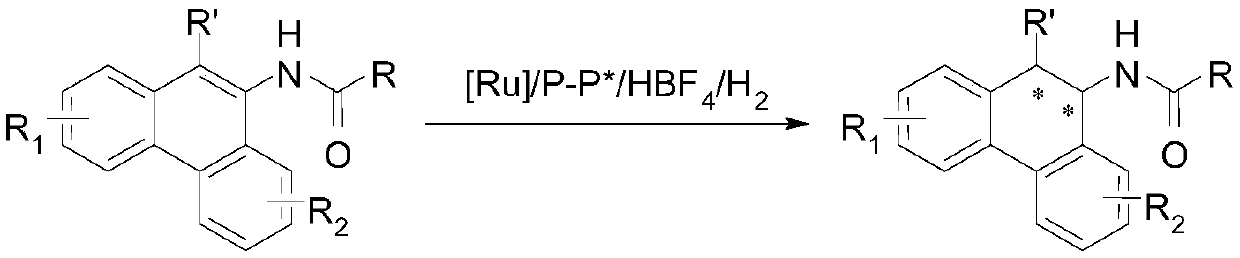
![Method for synthesizing chiral dihydro-6H-benzpyrole-[2,1-c][1,4]-benzodiazepine-6-ketone Method for synthesizing chiral dihydro-6H-benzpyrole-[2,1-c][1,4]-benzodiazepine-6-ketone](https://images-eureka.patsnap.com/patent_img/e6d181c0-4f2b-4590-96d9-77acc6e90e6f/FDA0000138255680000011.png)
![Method for synthesizing chiral dihydro-6H-benzpyrole-[2,1-c][1,4]-benzodiazepine-6-ketone Method for synthesizing chiral dihydro-6H-benzpyrole-[2,1-c][1,4]-benzodiazepine-6-ketone](https://images-eureka.patsnap.com/patent_img/e6d181c0-4f2b-4590-96d9-77acc6e90e6f/BDA0000138255690000021.png)
![Method for synthesizing chiral dihydro-6H-benzpyrole-[2,1-c][1,4]-benzodiazepine-6-ketone Method for synthesizing chiral dihydro-6H-benzpyrole-[2,1-c][1,4]-benzodiazepine-6-ketone](https://images-eureka.patsnap.com/patent_img/e6d181c0-4f2b-4590-96d9-77acc6e90e6f/BDA0000138255690000051.png)
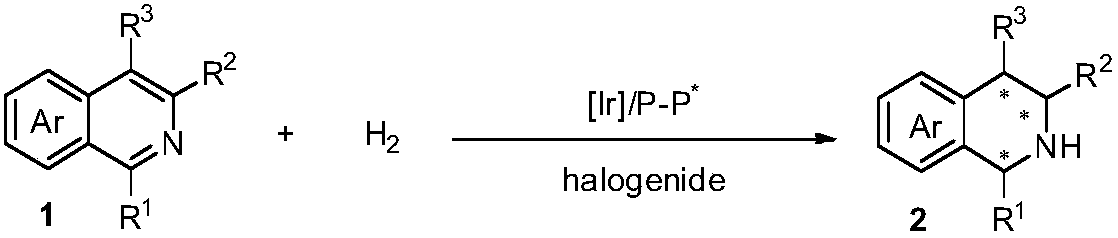
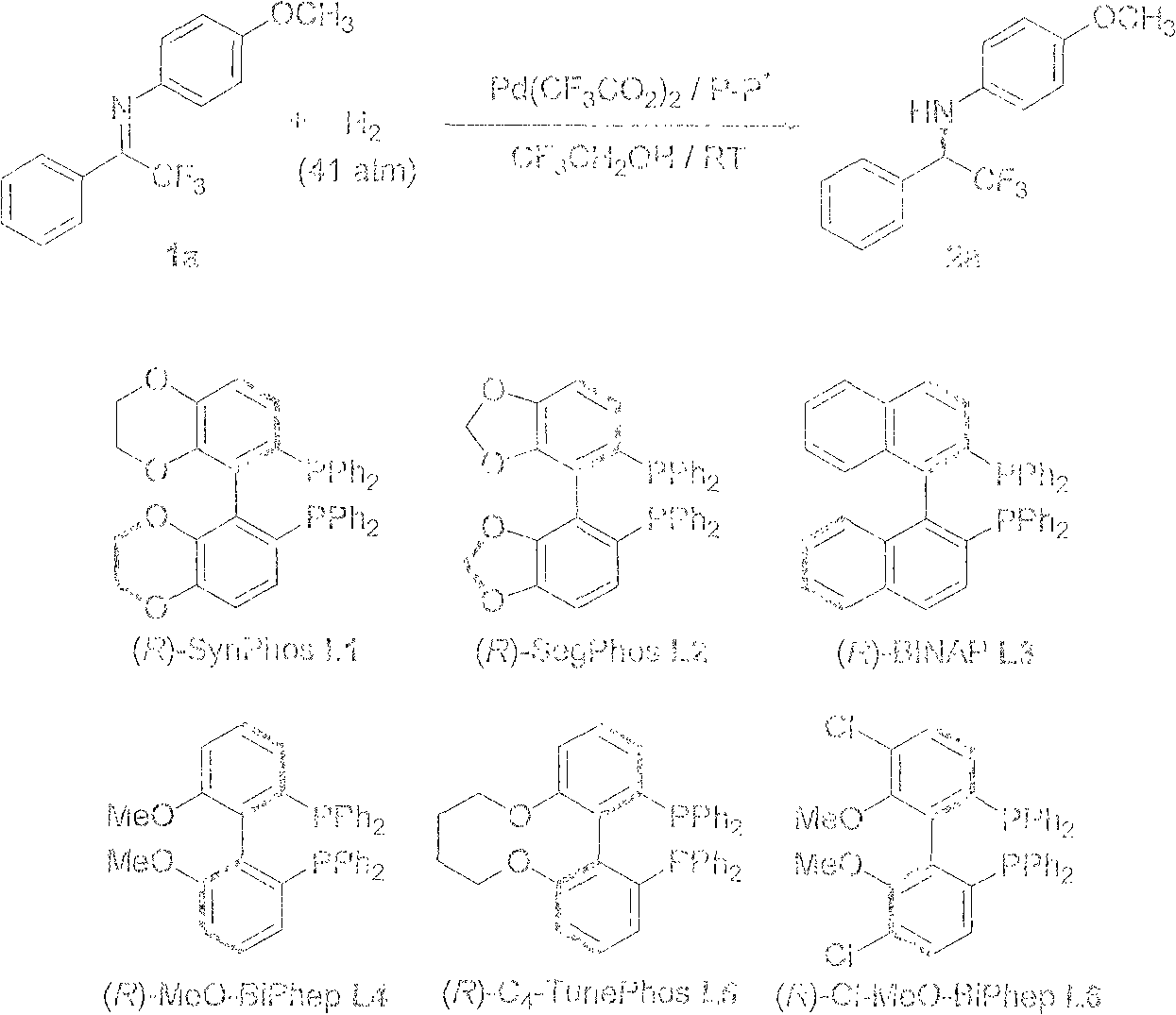
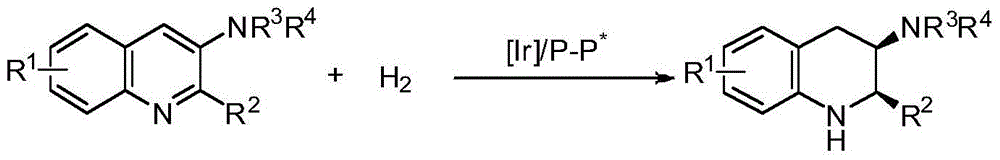
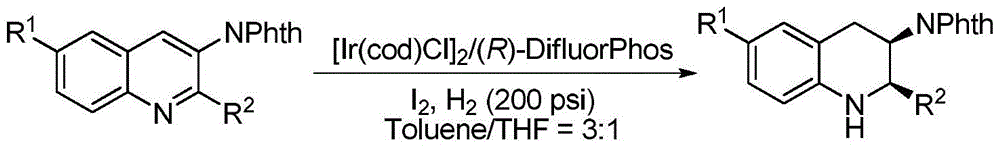
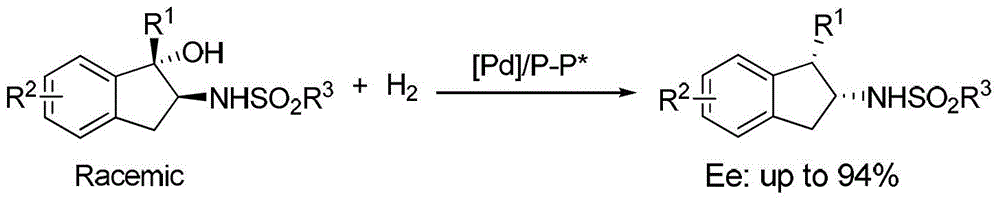
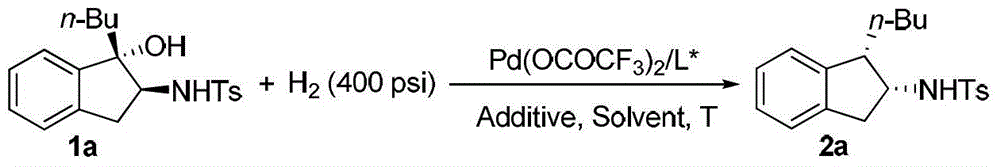
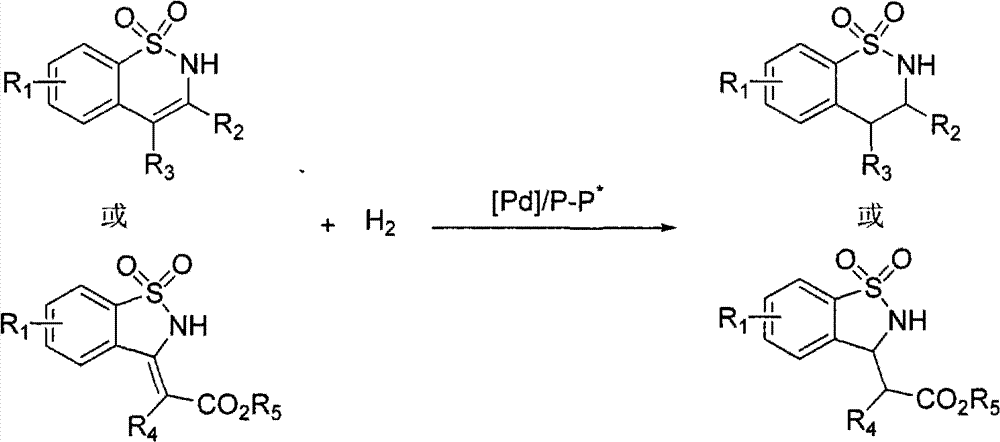
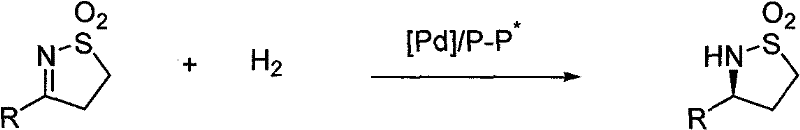
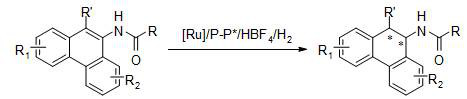




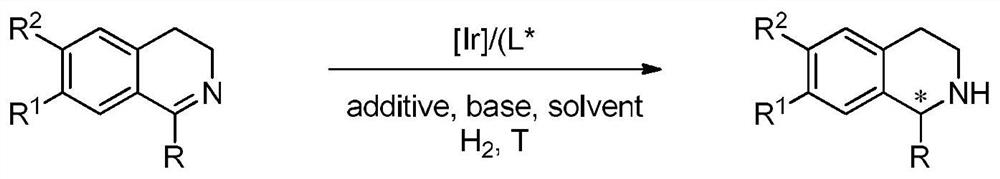
![A method for the synthesis of chiral amines by iridium-catalyzed asymmetric hydrogenation of pyrrole/indolo[1,2-a]quinoxaline A method for the synthesis of chiral amines by iridium-catalyzed asymmetric hydrogenation of pyrrole/indolo[1,2-a]quinoxaline](https://images-eureka.patsnap.com/patent_img/9b3bc703-2bc9-4d51-92bd-049e3e41db7f/BDA0001474915600000021.png)
![A method for the synthesis of chiral amines by iridium-catalyzed asymmetric hydrogenation of pyrrole/indolo[1,2-a]quinoxaline A method for the synthesis of chiral amines by iridium-catalyzed asymmetric hydrogenation of pyrrole/indolo[1,2-a]quinoxaline](https://images-eureka.patsnap.com/patent_img/9b3bc703-2bc9-4d51-92bd-049e3e41db7f/BDA0001474915600000022.png)
![A method for the synthesis of chiral amines by iridium-catalyzed asymmetric hydrogenation of pyrrole/indolo[1,2-a]quinoxaline A method for the synthesis of chiral amines by iridium-catalyzed asymmetric hydrogenation of pyrrole/indolo[1,2-a]quinoxaline](https://images-eureka.patsnap.com/patent_img/9b3bc703-2bc9-4d51-92bd-049e3e41db7f/BDA0001474915600000031.png)