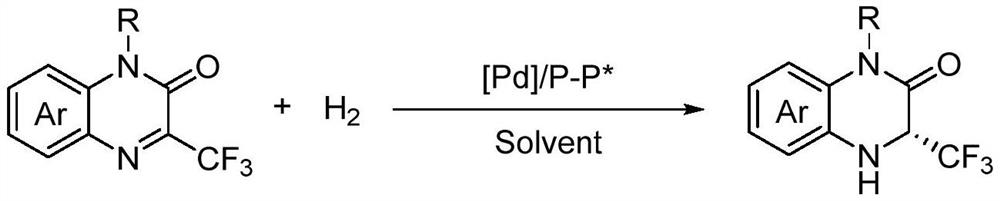
A method for palladium-catalyzed asymmetric hydrogenation of chiral 3-trifluoromethyl-3,4-dihydroquinoxalinone
A technology of trifluoromethylquinoxalinone and dihydroquinoxalinone, which is applied in the field of asymmetric hydrogenation synthesis, and achieves the effects of simple and practical reaction operation, convenient preparation and convenient separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-8
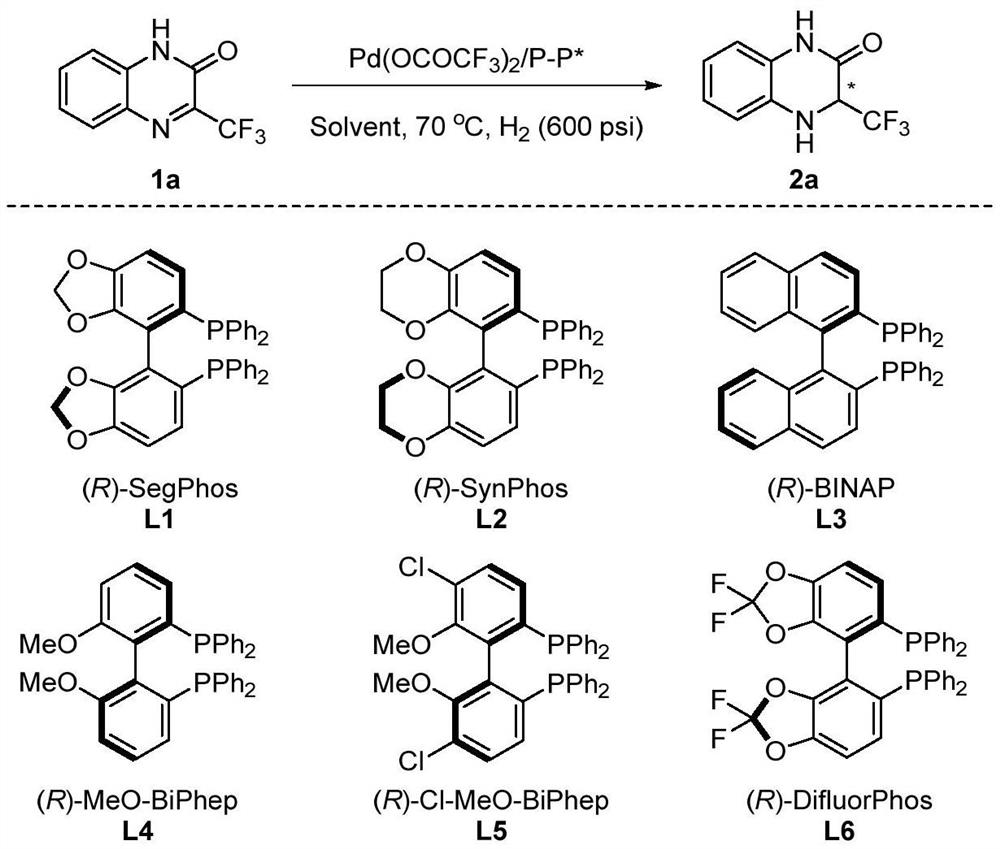
[0033] Condition optimization
[0034] Palladium (0.0025 mmol, 0.85 mg) and chiral ligand (0.003 mmol) were investigated in the reaction flask, and 1 ml of acetone was added after browning, and stirred at room temperature for 1 hour. Then, concentrated in vacuo, 3 ml of the organic solvent was added under nitrogen, and the solution was transferred to a reaction kettle presented with a substrate 1a (27 mg, 0.125 mmol), and hydrogen 600 psi was transferred to 60 ° C for 48 hours. Hydrogen is slowly released, and the direct column chromatography is removed from the solvent to obtain a pure product, alter the type of the organic solvent and the chiral bisphosphine ligand in the reaction, the temperature of the reaction, and the specific structure (Table 1), reaction and matching The body structure is as follows:
[0035]
[0036] The enantiomeric excess of the product was determined by a chromatographic chromatography, which is shown in Table 1.
[0037] Table 1. Asymmetric hydridin...
Embodiment 9-23
[0041] Palladium catalytic asymmetric hydrogenation synthesis of various chirofluoromethyl dihydroquinoxalin 2
[0042] Palladium (1.7 mg, 0.0050 mmol) and (R) -Segphos (3.4 mg, 0.0055 mmmol) were investred in the reaction flask, and 1 ml of acetone was added after nitrogen, and stirred at room temperature for 1 hour. Then, concentrated in vacuo, 3 ml of hexafluoropropyl alcohol was added under nitrogen, and the solution was transferred to a reaction kettle presented with a substrate (0.25 mmol), and hydrogen was transferred to 600 psi, and the reaction was reacted for 48 hours, and hydrogen was slowly released. The type of the reaction substrate is changed, as in Table 2. The pure product was separated from the direct column chromatography after removing the solvent, and the reaction formula was as follows:
[0043]
[0044] Table 2. Asymmetric hydrogenated substrate expansion of trifluorolinolinone
[0045]
[0046] The corresponding products obtained in Examples 9-23 are 2a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com