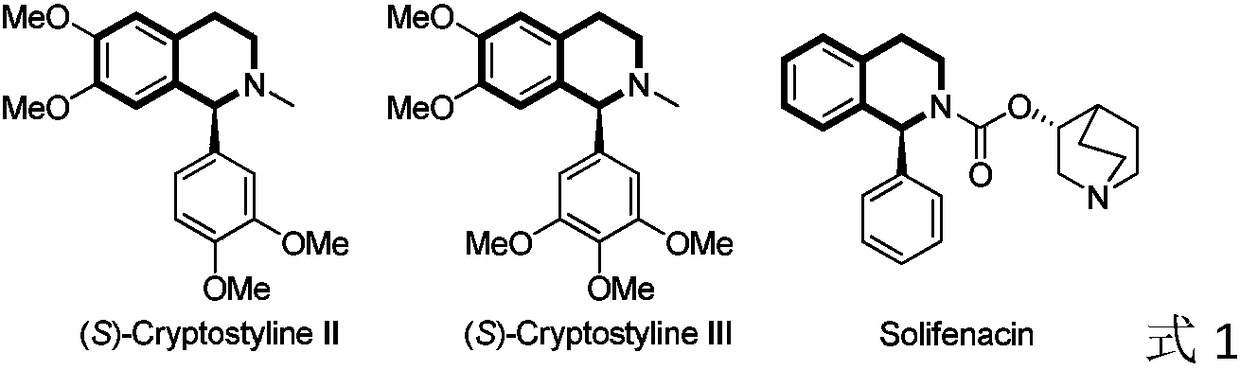
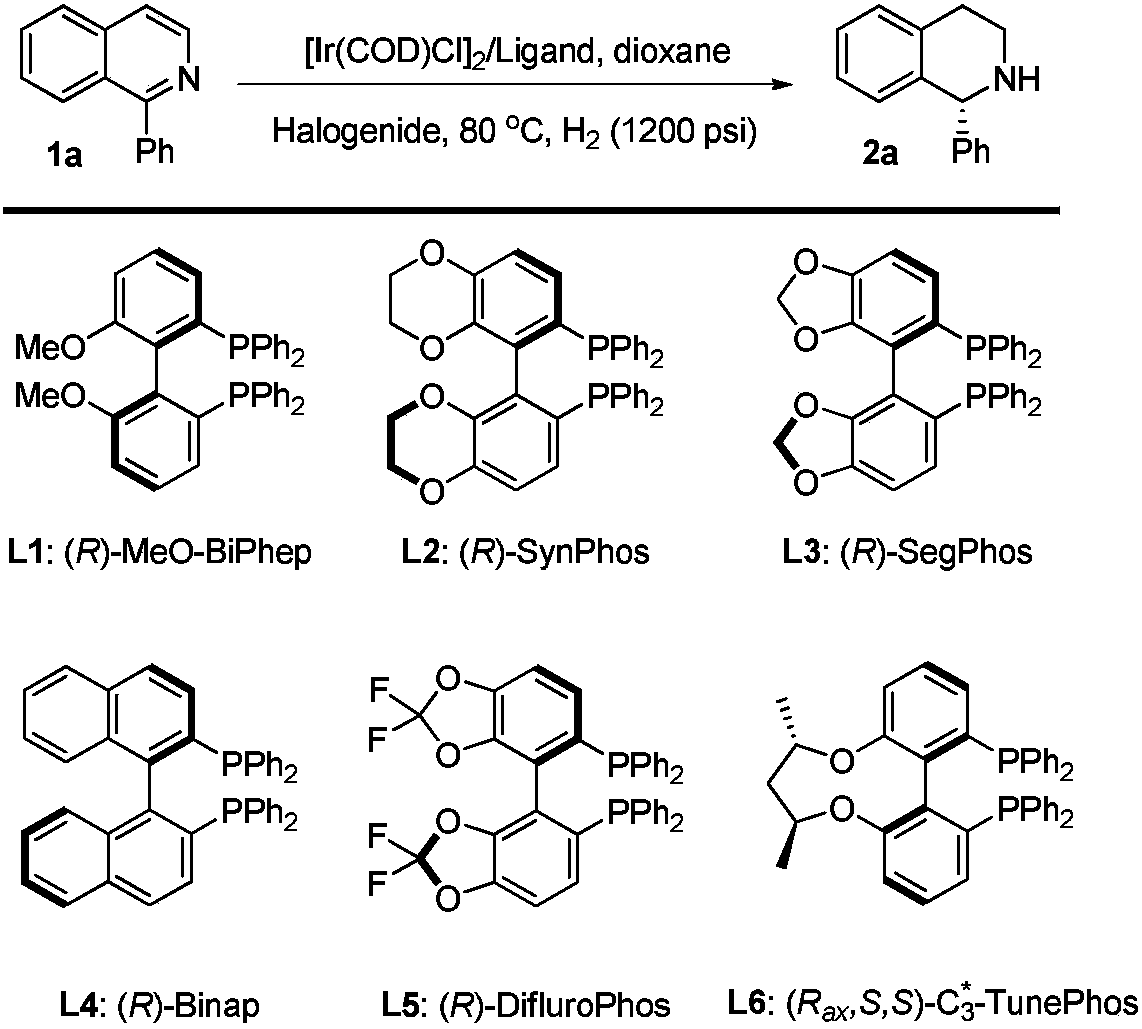
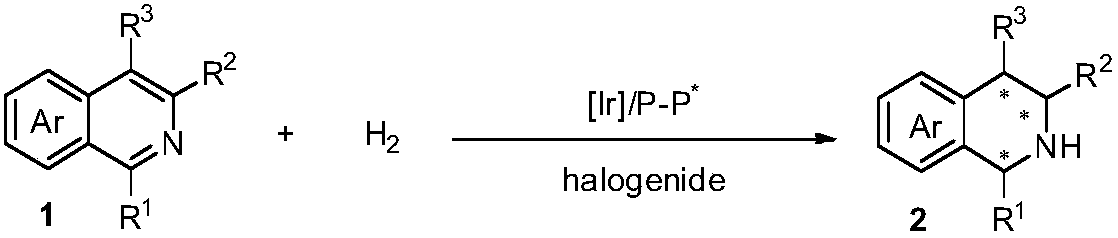Method for asymmetric hydrogenation of isoquinoline by activation of halogen bond
An isoquinoline and asymmetric technology is applied in the field of catalytic hydrogenation of fluorine-containing pyridine to synthesize chiral fluorine-containing piperidine derivatives, and achieves the effects of convenient preparation, high reactivity and enantioselectivity, and realizing chirality value-added.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: optimization of conditions
[0032] In a nitrogen-filled glove box, add (1,5-cyclooctadiene) iridium chloride dimer (0.0025 mmol, 1.7 mg) and chiral ligand (0.0055 mmol) to a reaction vial Add 1 ml of tetrahydrofuran, stir at room temperature for 10-30 minutes, then add halide and stir for 10-30 minutes, add isoquinoline (0.25 mmol) to the reaction flask with catalyst, and then add 2 ml of tetrahydrofuran. Put the reaction bottle into a stainless steel autoclave, feed hydrogen gas at 1200psi, and react at 80 degrees for 20-48 hours. Slowly release hydrogen, and add saturated Na to the system 2 CO 3 The aqueous solution was stirred for 10 minutes, then extracted three times with dichloromethane, the organic phases were combined and dried, and the solvent was removed by a rotary evaporator after direct column chromatography (the volume ratio of eluent sherwood oil and ethyl acetate was 10:1-5: 1) Isolate and obtain pure product, reaction formula and ligan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com