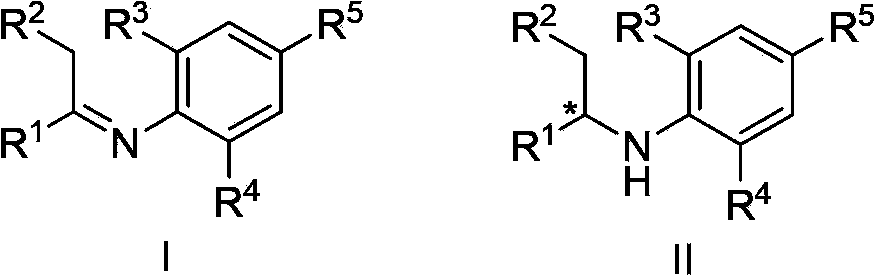
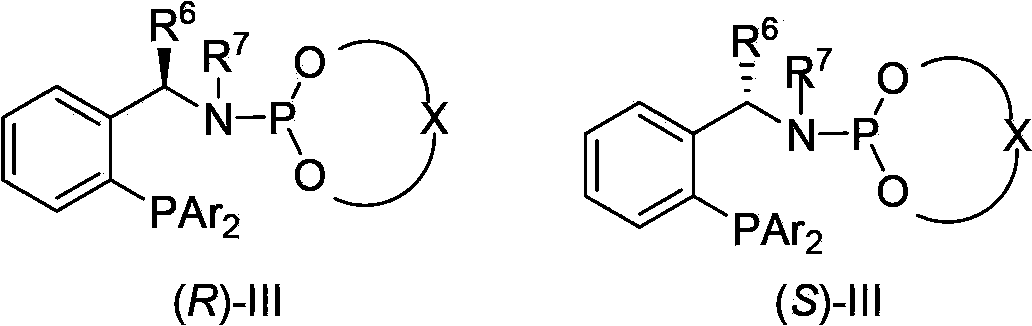
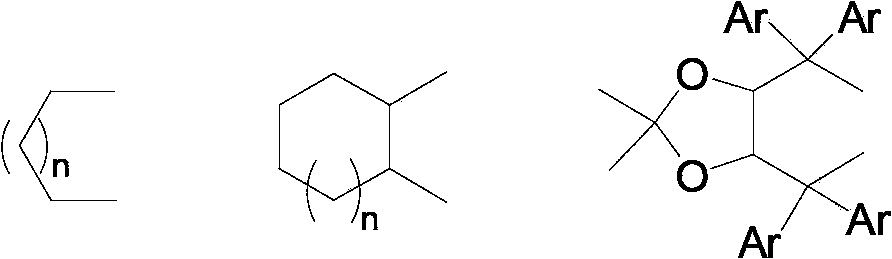
Asymmetric hydrogenation synthetic method of chiral aromatic amine compound with high steric hindrance
An aromatic amine, asymmetric technology, applied in the field of synthesis of high sterically hindered chiral aromatic amine compounds, can solve the problems of low reactivity, harsh reaction conditions, narrow substrate range, etc., and achieve high reactivity, mild reaction conditions and high activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Under nitrogen protection, [Ir(COD)Cl] 2 (0.0025mmol, 0.5mol%), chiral phosphine-phosphoramidite ligand (0.0055mmol, 1.1mol%) and KI (0.025mmol, 5.0mol%) were dissolved in dichloromethane (1.0mL), stirred at room temperature For 10 minutes, a solution of the substrate (E)-2,6-dimethyl-N-(1-phenylethylidene)aniline (0.5 mmol) in dichloromethane (1.0 mL) was added, and it was subjected to high pressure reaction In the kettle, the hydrogen gas was replaced 3 times, and then the hydrogen gas was fed to 20 atmospheres, and the reaction was carried out at room temperature for 24 hours. Slowly release hydrogen gas, remove the solvent and separate with a silica gel column to obtain the product 2,6-dimethyl N-(1-phenylethyl)aniline with a yield of 98% and ee of 97%. HPLC (chiralcel OJ-H, n-hexane / i-PrOH=90 / 10, 1.0mL / min, 254nm, 40°C):t R (major)=4.9min,t R (minor)=5.4min.[α] D 25 =-158(c=1.42in CHCl 3 ). 1 H NMR (400MHz, CDCl 3 ):δ=1.54(d,J=6.8Hz,3H),2.19(s,6H),3.22(br,1...
Embodiment 2
[0039] Using KI in Example 1 with I 2 Instead, the rest is the same as in Example 1, and the product 2,6-dimethyl-N-(1-phenylethyl)aniline is obtained in a 98% yield and 96% ee.
Embodiment 3
[0041] With the reaction condition H among the embodiment 1 2 The pressure was changed to 60 atmospheres, and the rest was the same as in Example 1. The product 2,6-dimethyl N-(1-phenylethyl)aniline was reacted with a yield of 98% and 97% ee.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com