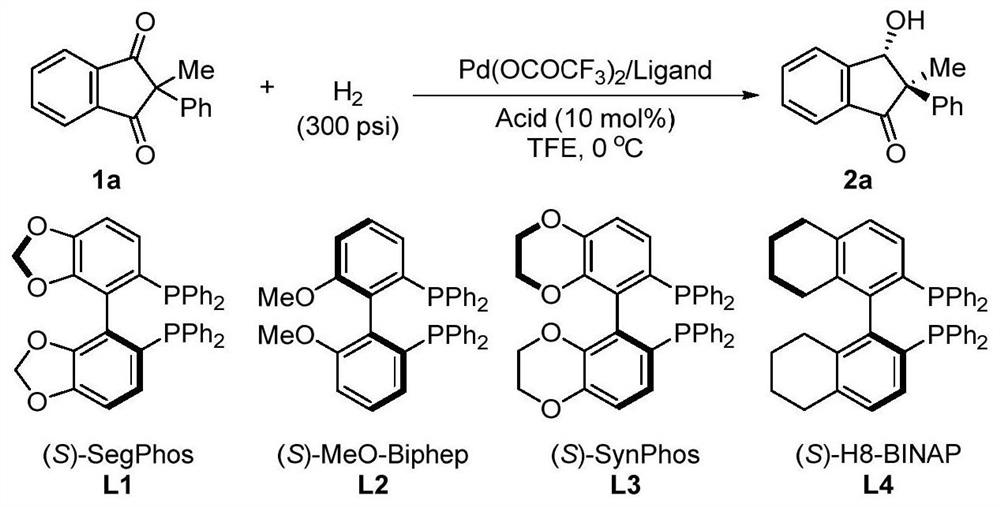
A method for palladium-catalyzed asymmetric hydrogenation of 1,3-diketones to synthesize β-hydroxy ketones
An asymmetric, palladium-catalyzed technology, applied in chemical instruments and methods, preparation of organic compounds, catalytic reactions, etc., to achieve the effects of convenient preparation, mild reaction conditions, and simple and practical reaction operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
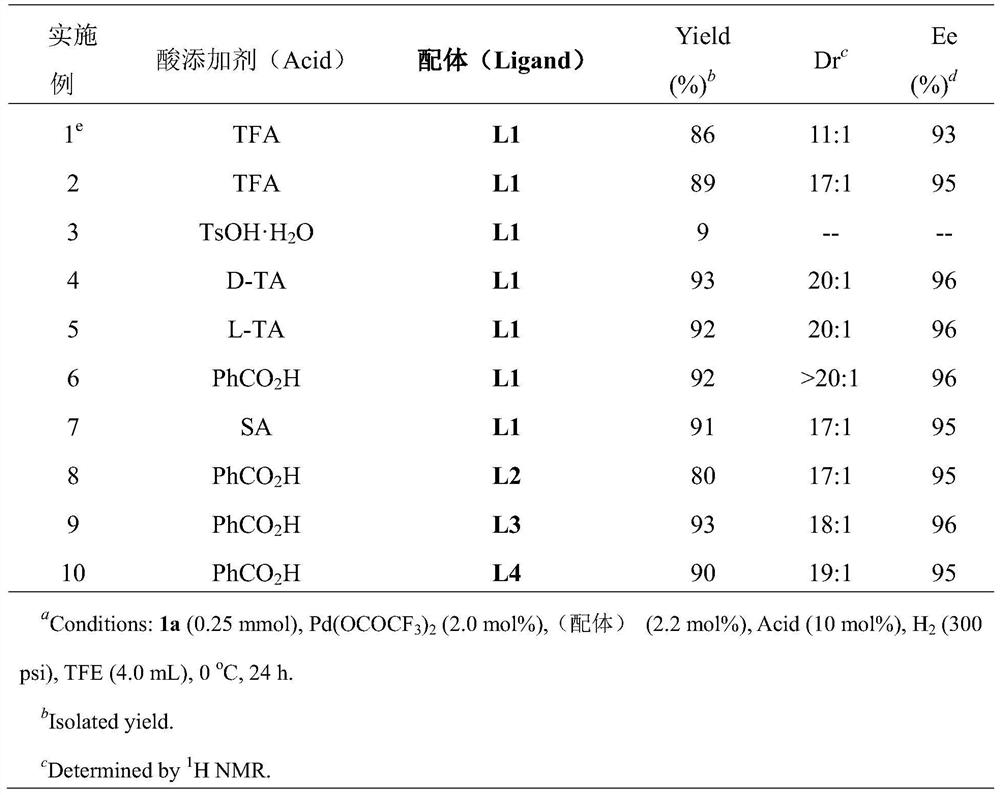
[0028] Optimization of hydrogenation reaction conditions
[0029] Under a nitrogen atmosphere, put the palladium precursor (1mol%-5mol% of the substrate amount) and the chiral bisphosphine ligand (1mol%-12mol% of the substrate amount) into the reaction flask, add acetone (1.0-2.0mL) After stirring at room temperature for 1 h, the acetone was removed under reduced pressure, and then the catalyst was brought into the glove box, and the catalyst was dissolved with trifluoroethanol and dichloromethane as solvents used for hydrogenation reaction, and transferred to a medium containing acid additive (1.0-2.0% of the amount of substrate used). equiv.) and substrate 0.2mmol), then put the reaction bottle into the reaction kettle, feed hydrogen (100psi-1000psi), and react for 12-150 hours at 0-80°C; release hydrogen, reduce Remove the solvent under pressure, add aqueous sodium bicarbonate, extract with DCM, combine the organic layers, remove the solvent under reduced pressure, and sepa...
Embodiment 2
[0036] Synthesis of Chiral β-Hydroxyketone 2 by Hydrogenation Desymmetry of 1,3-Diketone 1
[0037] Under a nitrogen atmosphere, put the palladium precursor (1mol%-5mol% of the substrate amount) and the chiral bisphosphine ligand (1mol%-12mol% of the substrate amount) into the reaction flask, add acetone (1.0-2.0mL) After stirring at room temperature for 1 h, the acetone was removed under reduced pressure, and then the catalyst was brought into the glove box, and the catalyst was dissolved with the solvent used in the hydrogenation reaction, and transferred to a solvent containing additive (1.0-2.0 equiv. of substrate dosage) and substrate 0.2mmol ) into the reaction bottle, and then put the reaction bottle into the reaction kettle, pass in hydrogen gas (100psi-1000psi), react at 0-80°C for 12-150h hours; release hydrogen gas, remove the solvent under reduced pressure, add sodium bicarbonate Aqueous solution, DCM extraction, combine the organic layers, remove the solvent under...
Embodiment 3
[0041] Synthesis of Chiral β-Hydroxyketone 4 by Hydrogenation Desymmetry of 1,3-Diketone 3
[0042] Under a nitrogen atmosphere, put the palladium precursor (1mol%-5mol% of the substrate amount) and the chiral bisphosphine ligand (1mol%-12mol% of the substrate amount) into the reaction flask, add acetone (1.0-2.0mL) After stirring at room temperature for 1 h, the acetone was removed under reduced pressure, and then the catalyst was brought into the glove box, and the catalyst was dissolved with the solvent used in the hydrogenation reaction, and transferred to a solvent containing additive (1.0-2.0 equiv. of substrate dosage) and substrate 0.2mmol ) into the reaction bottle, and then put the reaction bottle into the reaction kettle, pass in hydrogen (100psi-1000psi), react at 0-80°C for 12-150 hours; release hydrogen, remove the solvent under reduced pressure, add sodium bicarbonate Aqueous solution, DCM extraction, combine organic layer, remove solvent under reduced pressure,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com