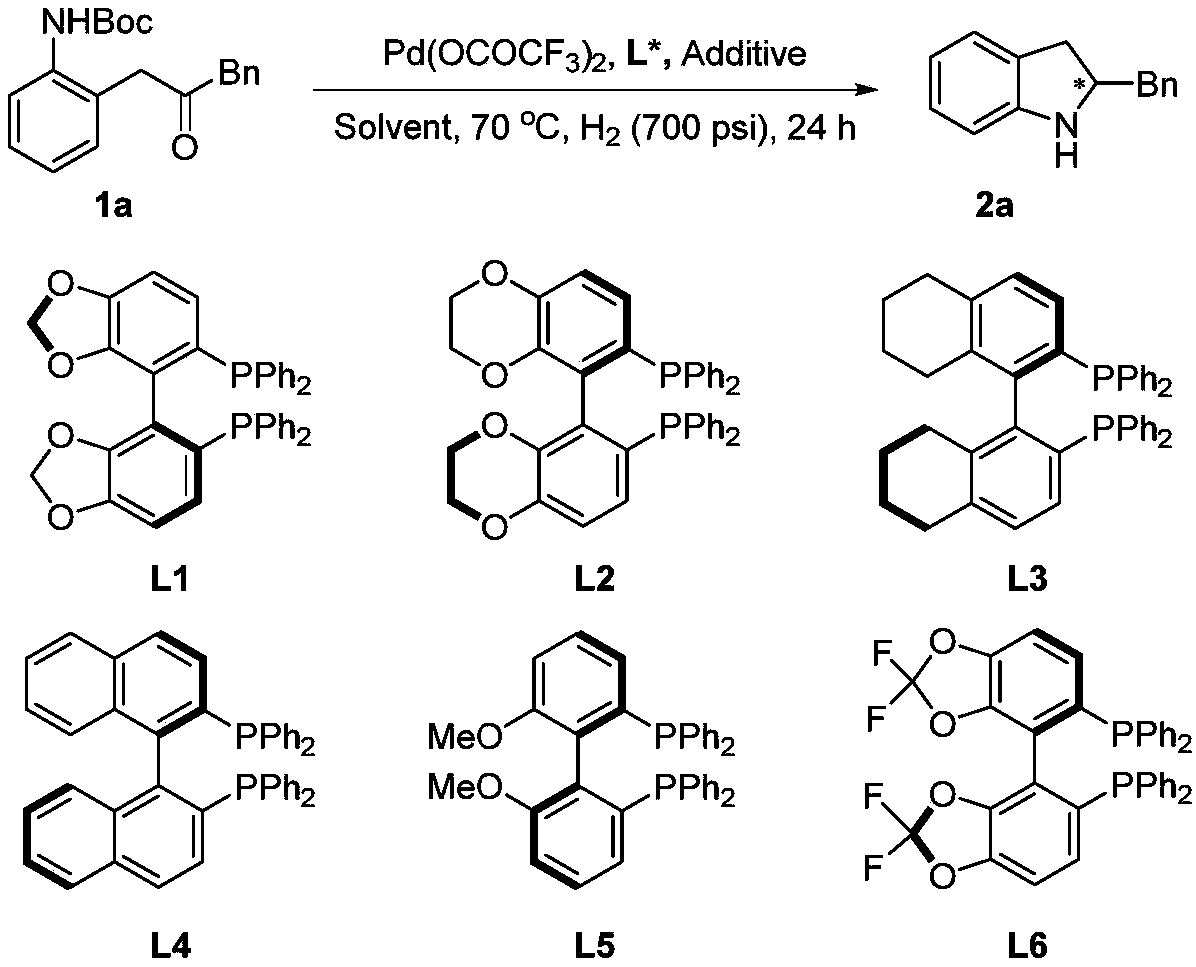
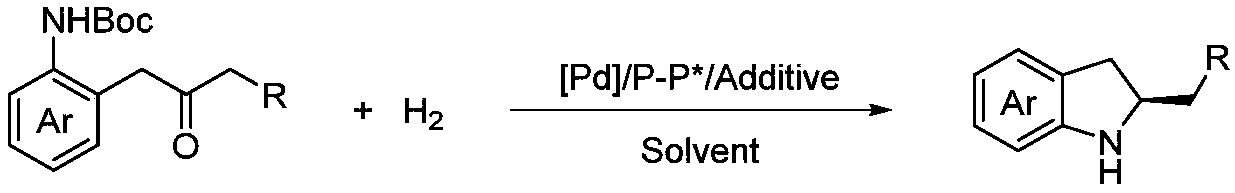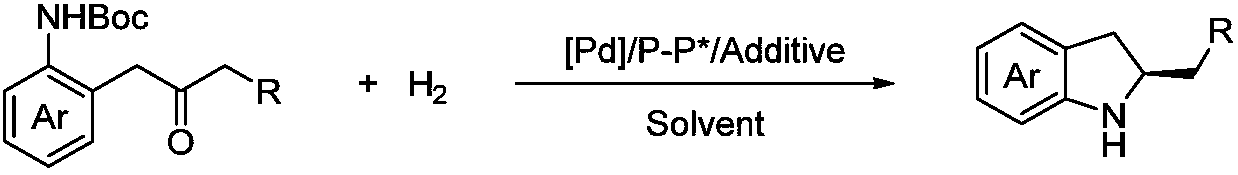Method for synthesizing chiral indoline through palladium catalyzed asymmetric hydrogenation of indole generated in situ
An in-situ generation and chemical synthesis technology, which is applied in the field of indole synthesis of chiral indolines, can solve the problems of difficulty and instability in indole synthesis, and achieves the effects of simple and practical reaction operation, convenient preparation and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-16
[0025] Optimization of hydrogenation reaction conditions
[0026] Under nitrogen atmosphere, drop into palladium precursor (1mol%-5mol% of substrate consumption) and chiral bisphosphine ligand (1.1mol%-5.5mol% of substrate consumption) in reaction bottle, add acetone (1.0-2.0 mL), stirred at room temperature for 1 h, and then removed acetone under reduced pressure, then brought the catalyst into the glove box, dissolved the catalyst with the organic solvent used for hydrogenation, and transferred it to a mixture containing additives (1.0-2.0 equiv. 0.2mmol reaction bottle, then put the reaction bottle into the reaction kettle, pass in hydrogen (10psi-1000psi), react at 0-80°C for 12-48 hours; release hydrogen, remove the solvent under reduced pressure, add carbonic acid Aqueous sodium hydrogen solution, extracted with DCM, combined the organic layers, removed the solvent under reduced pressure, and separated by column chromatography to obtain a pure product. 16 different exam...
Embodiment 17-30
[0033] Synthesis of Chiral Indoline 2 by Palladium-Catalyzed Asymmetric Hydrogenation of Indole Generated in Situ
[0034]Under nitrogen atmosphere, drop into palladium precursor (1mol%-5mol% of substrate consumption) and chiral bisphosphine ligand (1.1mol%-5.5mol% of substrate consumption) in reaction bottle, add acetone (1.0-2.0 mL), stirred at room temperature for 1h, and then removed acetone under reduced pressure, then brought the catalyst into the glove box, dissolved the catalyst with the solvent used for hydrogenation, and transferred it to a mixture containing additives (1.0-2.0 equiv. 0.25mmol reaction bottle, then put the reaction bottle into the reaction kettle, pass in hydrogen gas (10psi-1000psi), react at 0-80°C for 12-48 hours; release hydrogen gas, remove the solvent under reduced pressure, add hydrogen carbonate Sodium aqueous solution, DCM extraction, merging of organic layers, decompression to remove solvent, column chromatography separation to obtain pure ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com