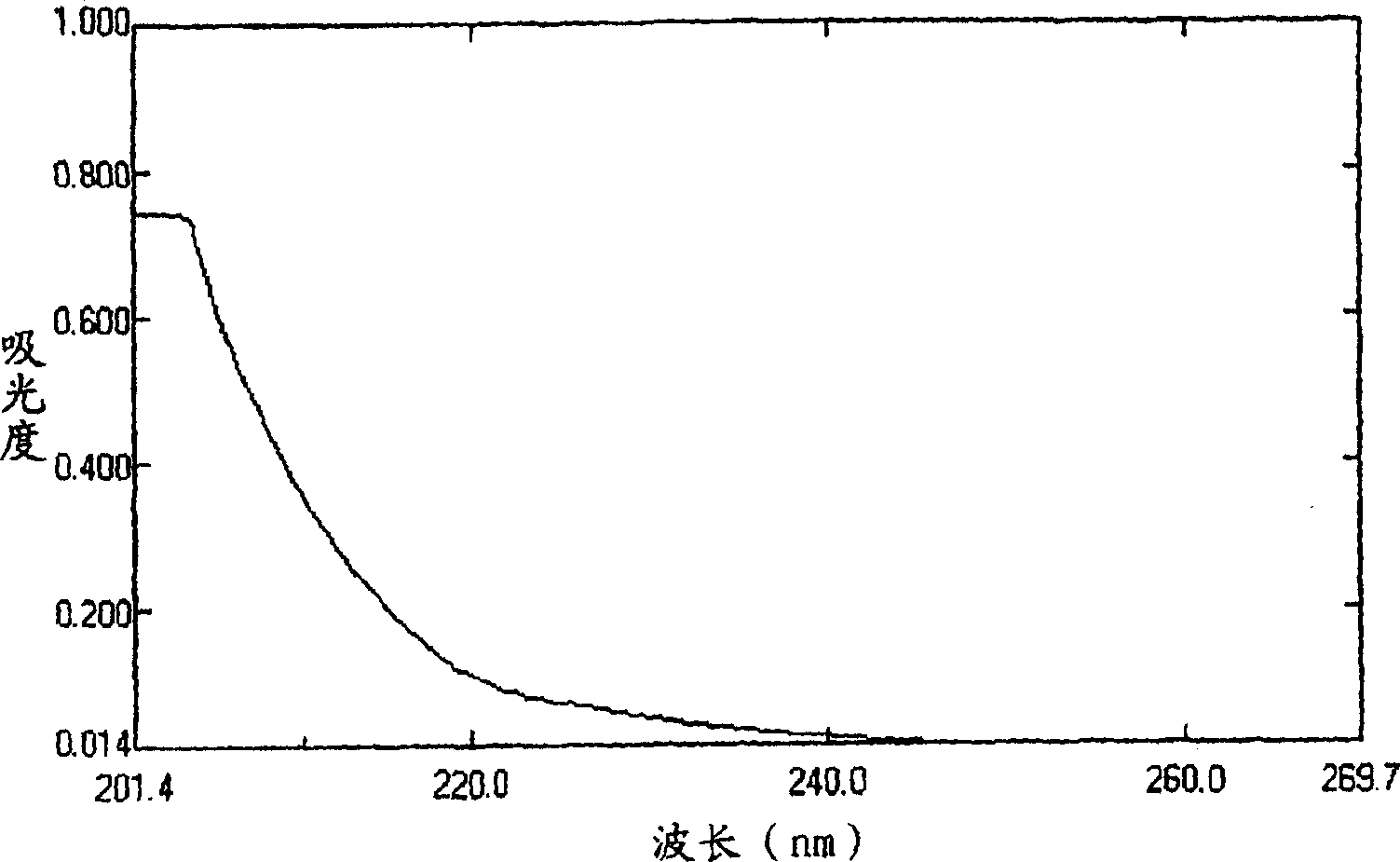
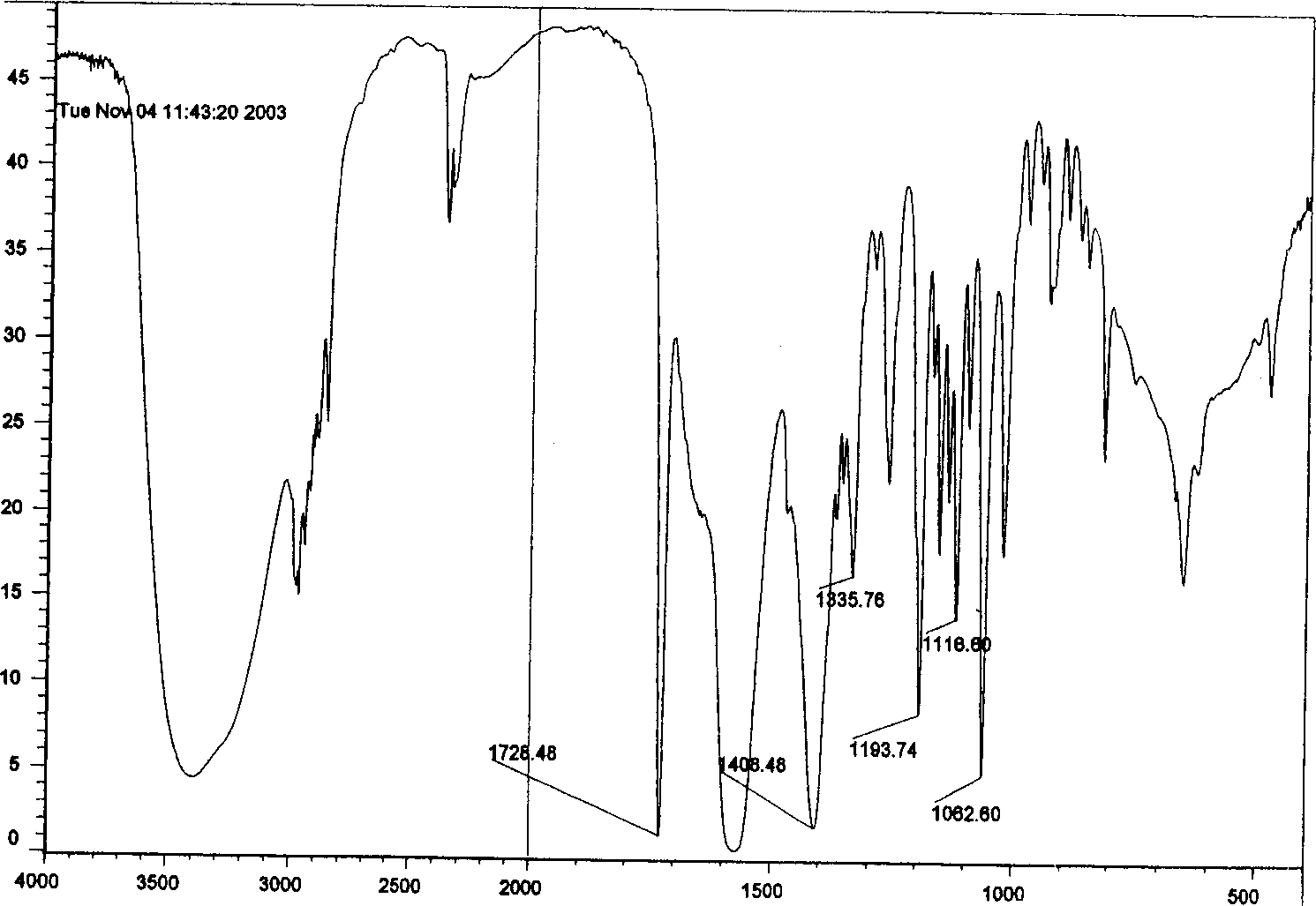
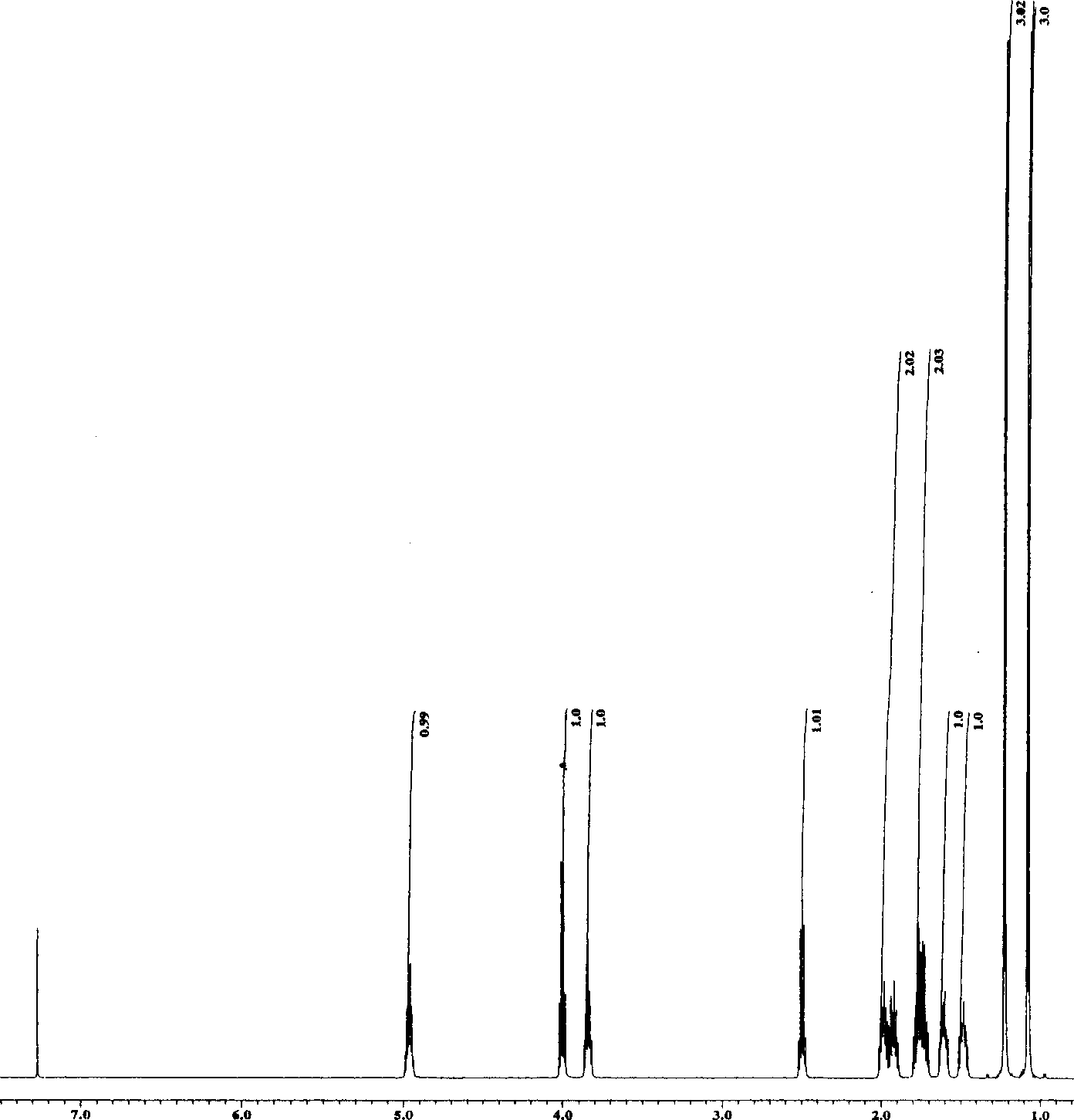
Compound of substituted nonane oxide-2-ketone, preparation method and application
A kind of compound, branched chain alkyl technology, applied in the field of substituted oxanenonane-2-one compounds and their preparation and use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Example 1 Fermentative Production and Separation and Purification of Compound I
[0080] Fermentation production
[0081] Toxin-producing bacteria
[0082] The toxin-producing bacteria used in this example to ferment and produce oxanonan-2-one compounds was Streptomyces flaviretus 18522 CGMCC 1022 strain isolated from soil samples in Xishuangbanna and identified through taxonomic research.
[0083] Fermentation culture of toxin-producing bacteria
[0084] According to the conventional method for culturing microorganisms, an appropriate amount of Streptomycesflavoretus 18522 CGMCC 1022 was inoculated onto the solid slant medium of Gaoshi Synthetic No. 1 agar, and cultured in an incubator at 28°C for 7 days.
[0085] Get the slant that has been cultivated for 7 days, pick an appropriate amount of spores and thalline with an inoculation needle, and inoculate into a medium containing 100 milliliters of seed culture solution (substrate composition: soybean powder 0.5%, solub...
Embodiment 2
[0109] Example 2 Tests for Inhibition of Proliferation, Cell Cycle Inhibition, and Apoptosis-Inducing Activity of Cancer Cells
[0110] Experimental samples and experimental methods
[0111] Preparation of the tested sample solution
[0112] The test sample is the pure compound I isolated and refined in Example 1 above. Accurately weigh an appropriate amount of sample, and prepare a solution with the required concentration with methanol for activity measurement.
[0113] Subculture of cell lines and cells
[0114] The activity test uses mammalian cancer cell lines such as human chronic myelogenous leukemia K562 cells, human colon cancer HCT-15 cells, human ovarian cancer A2780 cells, human lung cancer A549 cells, human cervical cancer HeLa cells and mouse breast cancer tsFT210 cells. All kinds of cells were fed with RPMI-1640 medium containing 10% FBS at 32°C (tsFT210 cells) or at 37°C (K562 cells, HCT-15 cells, A2780 cells, A549 cells and HeLa cells). Subculture in a carb...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com