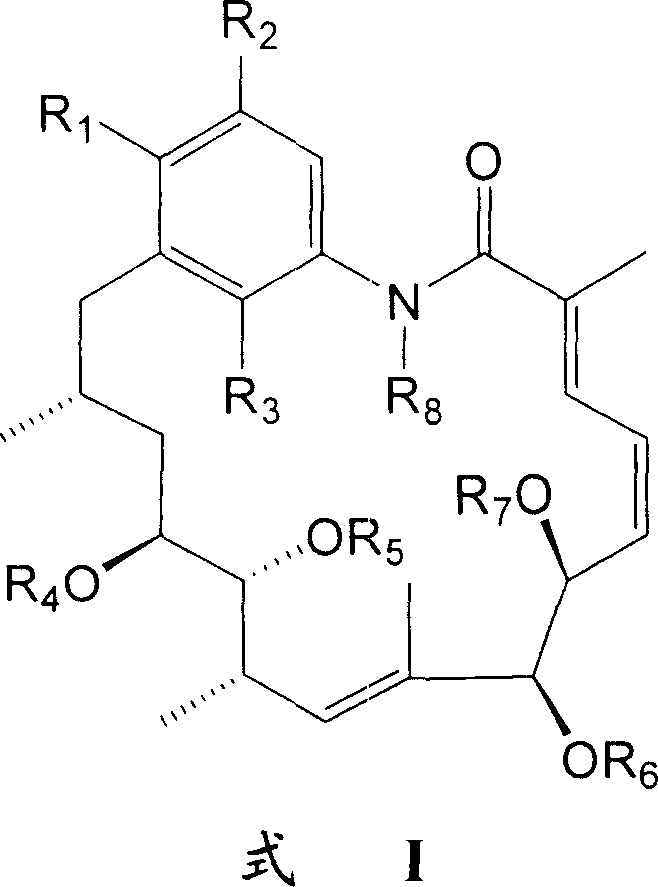
Fragrant geldanamycin derivative and its preparing method and use
A technique for geldanamycin and aromatization, which is applied in the field of aromatization of geldanamycin derivatives, and can solve problems such as benzoquinone reduction that have not yet been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Fermentative production and separation and purification of embodiment 1 compound 1
[0095] Fermentation production
[0096] Toxin-producing bacteria
[0097] The toxin-producing bacteria used for fermentative production of compound 1 in this example was isolated from soil samples collected from Xishuangbanna and identified as Streptomycespseudoverticillus strain YN17707 CGMCC No.1452 through taxonomic studies.
[0098] Fermentation culture of toxin-producing bacteria
[0099] According to the conventional method of cultivating microorganisms, an appropriate amount of Streptomycespseudoverticillus YN17707 CGMCC No.1452 strain was taken, inoculated into an eggplant-shaped flask on the Gaoshi Synthetic No. 1 agar solid slant medium, and activated for 7 days in an incubator at 28 degrees Celsius. Get the eggplant-shaped bottle slope that has been activated and cultivated for 7 days, add 15 milliliters of sterile water, scrape off the spores with an inoculation needle, ma...
Embodiment 2
[0109] The separation and purification of embodiment 2 compound 2
[0110] Get 30 grams of the chloroform extract obtained in the above-mentioned Example 1, dissolve it with an appropriate amount of chloroform, add 50 grams of silica gel G (200-300 mesh) produced by Qingdao Ocean Chemical Factory to mix the sample, add to the silica gel that is equipped with 300 grams of thin-layer chromatography. 60H (Qingdao Haiyang Chemical Factory) glass decompression column, carry out decompression column chromatography, use chloroform-methanol system (100:0→80:20) gradient elution, every 500ml is a fraction, and several fractions are obtained. share. According to the results of TLC detection and activity test, the corresponding fractions were combined to obtain the active component Fr-4 containing compound 2 (the elution fraction of chloroform-methanol 80:20). Dissolve the active component Fr-4 in an appropriate amount of chloroform, add an appropriate amount of silica gel G (200-300 me...
Embodiment 3
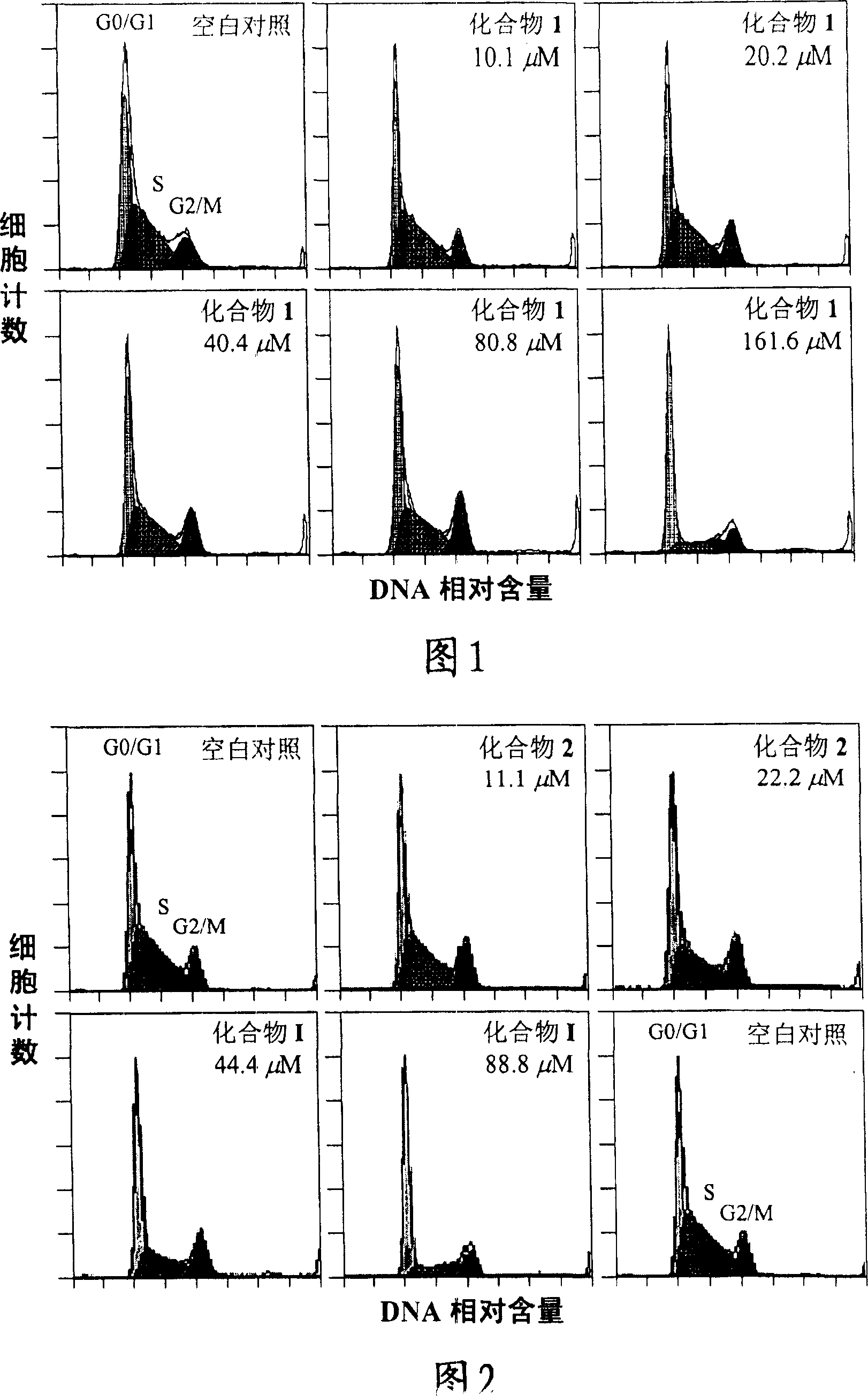
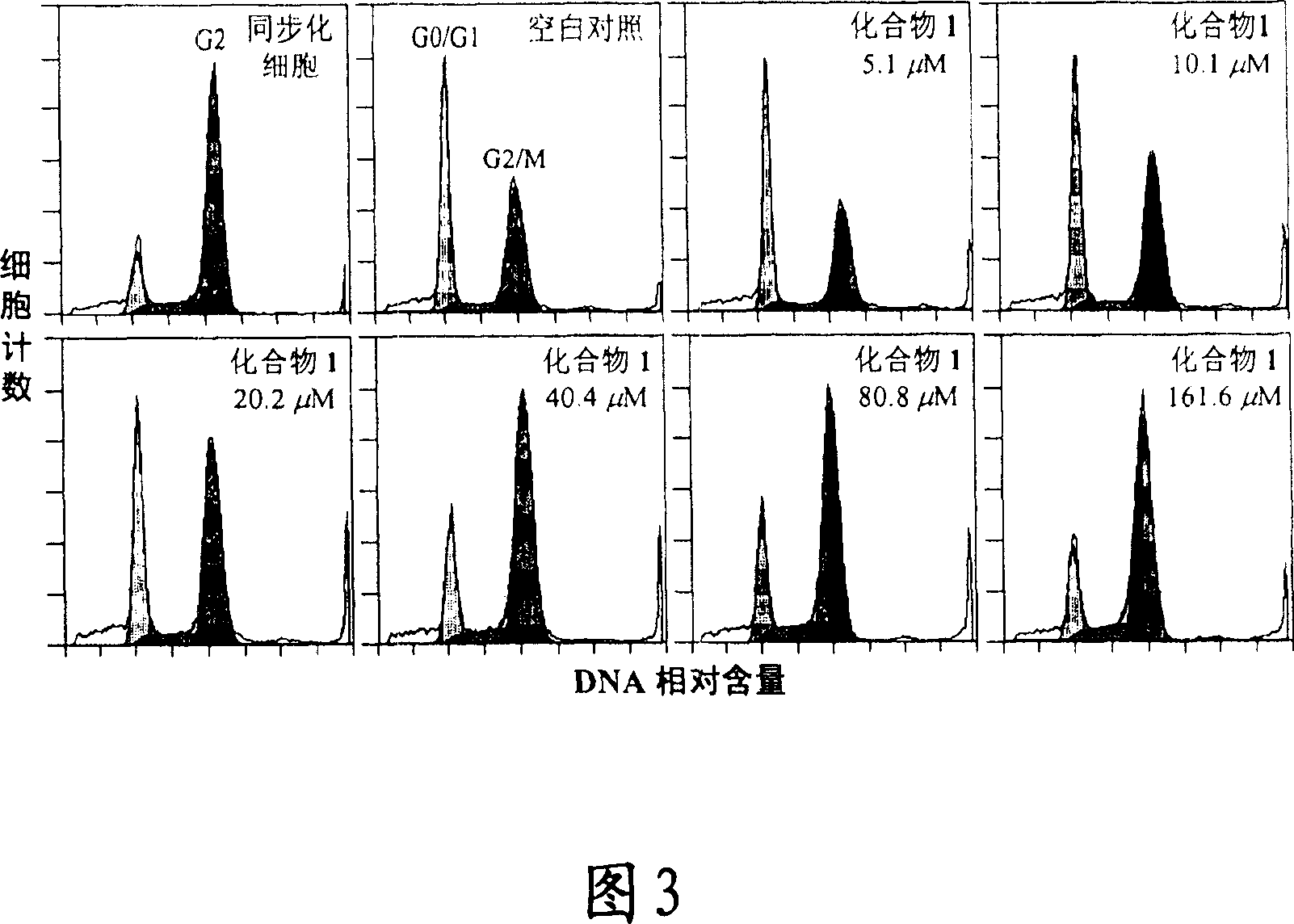
[0118] Example 3 Cell Cycle Inhibitory Activity Test of Compound 1 and Compound 2
[0119] Experimental samples and experimental methods
[0120] Preparation of the tested sample solution
[0121] The test samples were the pure compound 1 and compound 2 separated and refined in the above-mentioned example 1 and example 2. Accurately weigh an appropriate amount of sample, and use methanol to make a solution of the required concentration for testing the activity.
[0122] Subculture of cell lines and cells
[0123]The activity test adopts the temperature-sensitive mouse breast cancer tsFT210 cell line, and the cells are subcultured in RPMI-1640 medium containing 10% FBs at 32 degrees Celsius in a cell incubator filled with 5% carbon dioxide.
[0124] Flow Cytometry Activity Assay Method
[0125] asynchronous culture test
[0126] Take the tsFT210 cells in the logarithmic growth phase, and use fresh RPMI-1640 medium to prepare a density of 2×10 per ml 5 The cell suspension ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com