Quinolone analogue as well as preparation method and application thereof
A technology of analogues and quinolones, which is applied in the field of medicinal chemistry, can solve the problems such as no one discloses the preparation method of the compound, impurity control, etc., and achieve the effect of simple and easy preparation method, improved quality and improved safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1)
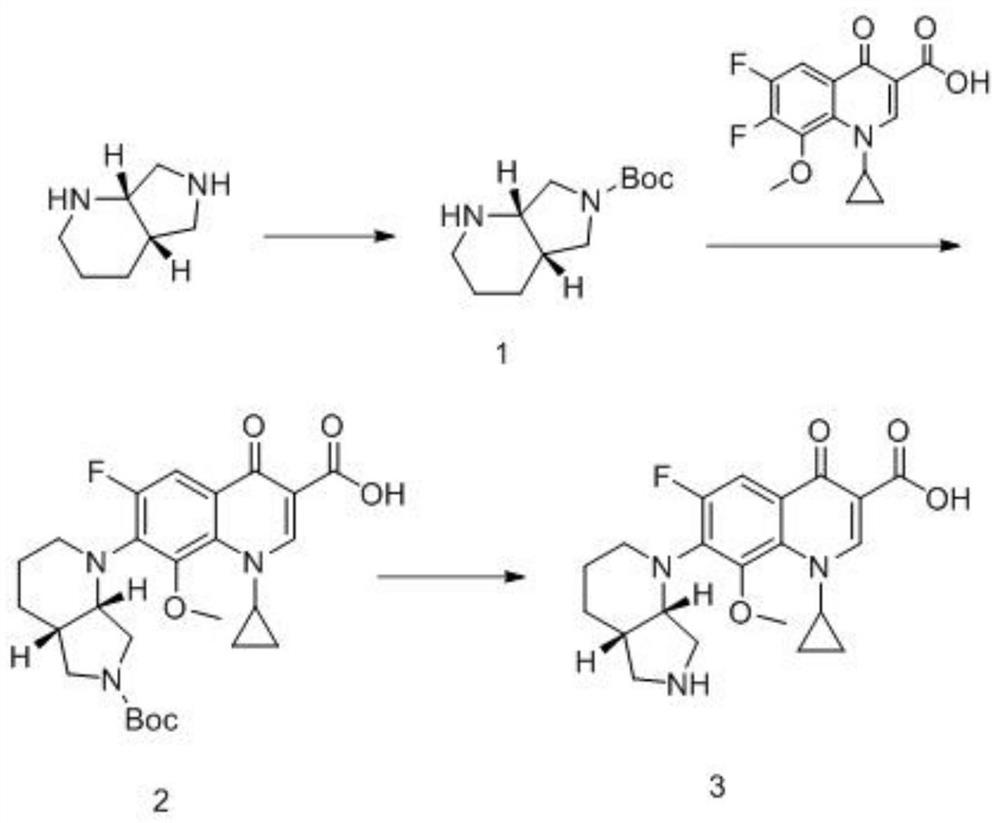
[0036] The structural formula of the quinolone analog to be prepared in this embodiment is as follows:
[0037] ,
[0038] Name it 1-cyclopropyl-7-(S,S-2,8-diazo-bicyclo[4.3.0]nonan-2-yl)-6-fluoro-8-methoxy-1, 4-Dihydro-4-oxo-3-quinolinecarboxylic acid, see the synthetic route figure 1 , including the following steps:
[0039] ① Preparation of compound 1, the reaction formula is as follows:
[0040] .
[0041] In a reaction flask, 10 g of S,S-2,8-diazo-bicyclo[4.3.0]nonane was dissolved in 40 mL of ethyl acetate, and then 8.8 g of triethylamine was added to the ethyl acetate to disperse evenly.
[0042] Dissolve 19g of di-tert-butyl dicarbonate in 200mL of ethyl acetate, and then add it dropwise into the reaction flask under stirring. The reaction temperature is 30-40°C. After the reaction, concentrate under reduced pressure (temperature 40°C-50°C, pressure -0.08~-0.1MPa).
[0043] The concentrated product was dissolved in 50mL of dichloromethane, separated and purif...
Embodiment 2)
[0060] All the other preparation methods of the quinolone analogs of the present embodiment are the same as in Example 1, except that:
[0061] In step ①, in a reaction flask, dissolve 10 g of S,S-2,8-diazo-bicyclo[4.3.0]nonane in 50 mL of tetrahydrofuran, and add 6.4 g of diethylamine to disperse evenly.
[0062] After dissolving 19g of di-tert-butyl dicarbonate in 20mL of tetrahydrofuran, it was added dropwise to the reaction system under stirring. The reaction temperature was 30-40°C. After the dropwise addition, continue to stir and react at 30-40°C for 4-5h.
[0063] After purification, 4.0 g of compound 1 was obtained as a solid.
Embodiment 3)
[0065] All the other preparation methods of the quinolone analogs of the present embodiment are the same as in Example 1, except that:
[0066] In step ①, in a reaction flask, dissolve 20 g of S,S-2,8-diazo-bicyclo[4.3.0]nonane in 40 mL of ethyl acetate, and add 14.3 g of N-methylimidazole to disperse evenly.
[0067] After dissolving 41.5g of di-tert-butyl dicarbonate in 20mL of ethyl acetate, it was added dropwise to the reaction system while stirring. The reaction temperature was 30-40°C.
[0068] After purification, 8.3 g of compound 1 was obtained as a solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com