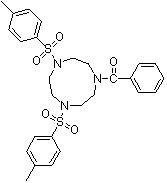
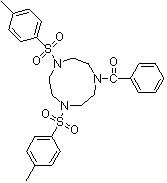
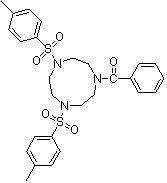
Synthesis method of 1,4-bis(p-toluenesulfonyl)-7-benzoyl trinitrocyclononane
A technology of benzoyl triazine and p-toluenesulfonyl, which is applied in the field of synthesis of 1,4-bis-7-benzoyl triazacyclononane, which can solve the problems of waste acid, low reaction yield and production efficiency Low-level problems, to achieve the effect of simple process control, avoid side reactions, and low production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] In a 2000ml four-necked flask equipped with an electric stirrer, a thermometer, a reflux condenser, and an oil bath heating pot, add 50g (0.097mol) N-benzoyl-O, O-p-toluenesulfonyl-diethanolamine, 37g (0.1mol) N,N'-bis(p-toluenesulfonyl)ethylenediamine, 500ml of toluene, 100ml of tap water, 45g (0.33mol) of anhydrous potassium carbonate, after adding the above materials, start to stir and heat up.
[0026] When the temperature rose to 90°C, the system boiled and there was reflux in the reflux condenser. Timing heat preservation and reflux, after heat preservation for 5 hours, after cooling, the lower water layer was separated, and the organic layer was washed with water until neutral. Dry over anhydrous sodium sulfate and transfer to a rotary evaporator. The solvent was decompressed by the oil pump, and a loose white irregular powder was obtained in the rotary evaporating bottle, which was poured out and weighed 41g. The liquid chromatography content is 93.21%, based ...
Embodiment 2
[0028] In a 2000ml four-necked flask equipped with an electric stirrer, a thermometer, a reflux condenser, and an oil bath heating pot, add 50g (0.097mol) N-benzoyl-O, O-p-toluenesulfonyl-diethanolamine, 37g (0.1mol) N,N'-di(p-toluenesulfonyl)ethylenediamine, 800ml xylene, 100ml tap water, 66g (0.48mol) anhydrous potassium carbonate, after adding the above materials, start to stir and heat up.
[0029] When the temperature rose to 98°C, the system boiled and there was reflux in the reflux condenser. Timing heat preservation and reflux, after heat preservation for 24 hours, after cooling, the lower water layer was separated, and the organic layer was washed with water until neutral. Dry over anhydrous sodium sulfate and transfer to a rotary evaporator. The oil pump decompresses and spins out the solvent, and loose white irregular powder is obtained in the rotary evaporating bottle, which is poured out and weighed to be 45g. The liquid chromatography content is 96.09%, and the...
Embodiment 3
[0031] In a 2000ml four-necked flask equipped with an electric stirrer, a thermometer, a reflux condenser, and an oil bath heating pot, add 50g (0.097mol) N-benzoyl-O, O-p-toluenesulfonyl-diethanolamine, 37g (0.1mol) N,N'-di(p-toluenesulfonyl)ethylenediamine, 800ml xylene, 100ml tap water, 50g (0.47mol) anhydrous sodium carbonate, after adding the above materials, start to stir and heat up.
[0032] When the temperature rose to 98°C, the system boiled and there was reflux in the reflux condenser. Timing heat preservation and reflux, after heat preservation for 24 hours, after cooling, the lower water layer was separated, and the organic layer was washed with water until neutral. Dry over anhydrous sodium sulfate and transfer to a rotary evaporator. The oil pump decompresses and spins out the solvent, and loose white irregular powder is obtained in the rotary evaporating bottle, which is poured out and weighed to be 33g. The liquid chromatography content is 89.56%, and the mo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com