2-nitro-2-azaadamantane-4, 6, 8-triol trinitrate and preparation method thereof
A technology of triol trinitrate and azaadamantane, applied in the direction of nitrated pentaerythritol composition, organic chemistry, etc., can solve the problems that have not been reported, and achieve the effects of high yield, easy scale-up preparation, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] Synthetic method of the present invention, its concrete steps are as follows:
[0034] (1) Dissolve bicyclo[3.3.1]nonane-2,6-dione and p-toluenesulfonyl hydrazide in methanol, an organic solvent, and stir at 65-75°C for 2-6 hours to synthesize 9- Hydroxybicyclo[3.3.1]nonane-2,6-diylidenebis(4-methylbenzenesulfonylhydrazide);
[0035] (2) Add diisopropylamine and n-butyllithium to the organic solvent tetrahydrofuran sequentially at low temperature, and then add 9-hydroxybicyclo[3.3.1]nonane-2,6-diylidene bis(4-methylbenzenesulfonate Hydrazide) was added to the reaction solvent in batches, stirred at -68 to -78°C for 18 to 24 hours, and an elimination reaction occurred to synthesize bicyclo[3.3.1]nonane-2,6-dien-9-ol;
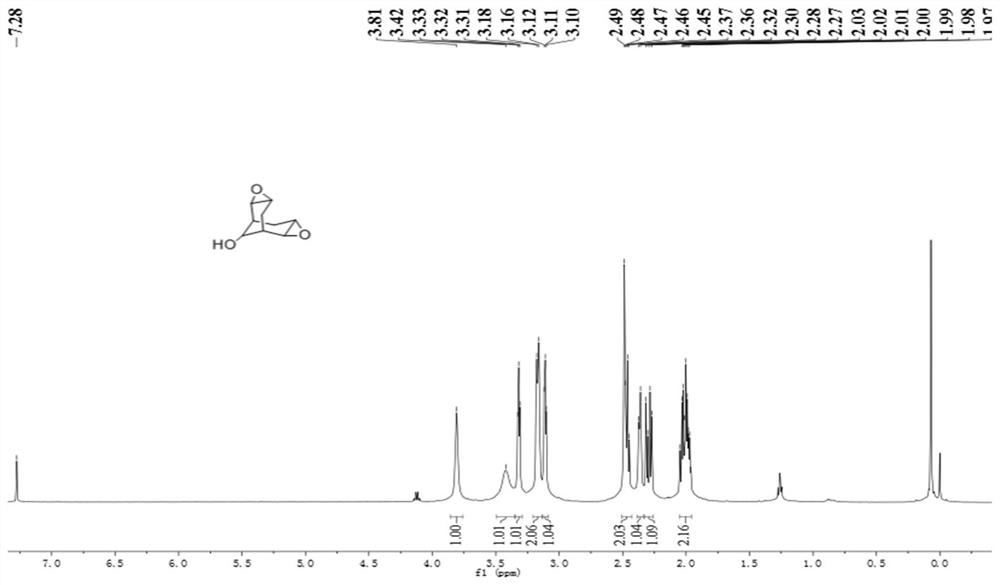
[0036] (3) Dissolve bicyclo[3.3.1]nonan-2,6-dien-9-ol and m-chloroperoxybenzoic acid in the organic solvent dichloromethane, stir at 25~40°C for 24~36h, and Synthesis of 3,8-dioxa-tetracyclo[4.4.1.0 by epoxidation reaction 2,4 .0 7,9 ]undecan-11-ol;
...
Embodiment 1
[0041] (1) Preparation of 9-hydroxybicyclo[3.3.1]nonane-2,6-diylidene bis(4-methylbenzenesulfonylhydrazide)
[0042]
[0043]Add 504mg (3.00mmol) of 9-hydroxybicyclo[3.3.1]nonane-2,6-dione into 25mL of methanol, then add 1.34g (7.2mmol) of p-toluenesulfonyl hydrazide, heat up to 68°C for reflux reaction 4h. The solvent was distilled off under reduced pressure, washed with cold absolute ethanol, and dried in vacuo to obtain 1.36 g of a white solid. Yield 90%.
Embodiment 2
[0045] (2) Preparation of bicyclo[3.3.1]nonane-2,6-dien-9-ol
[0046]
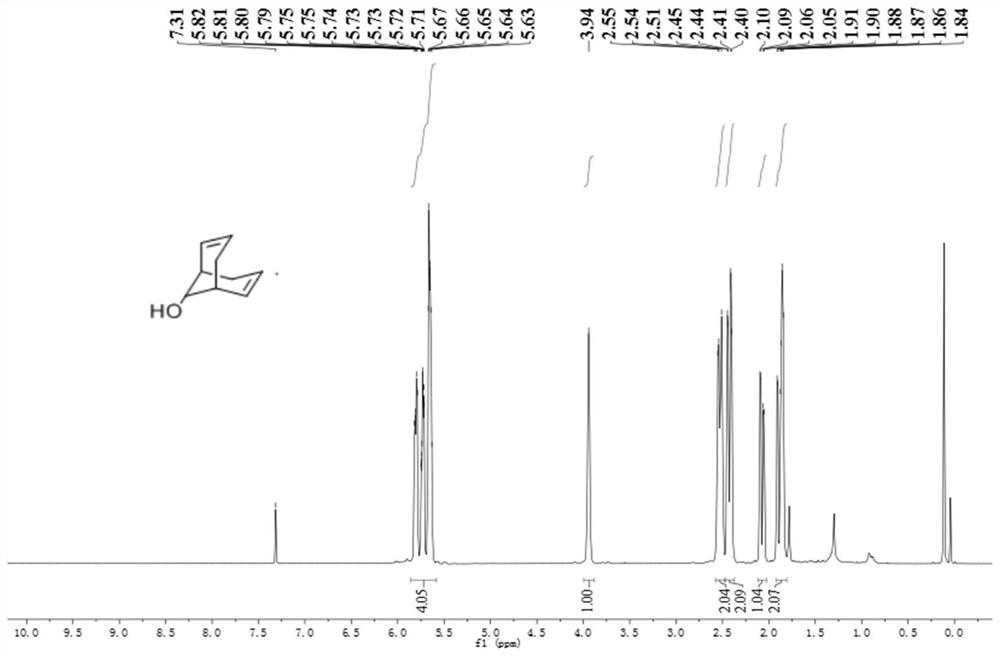
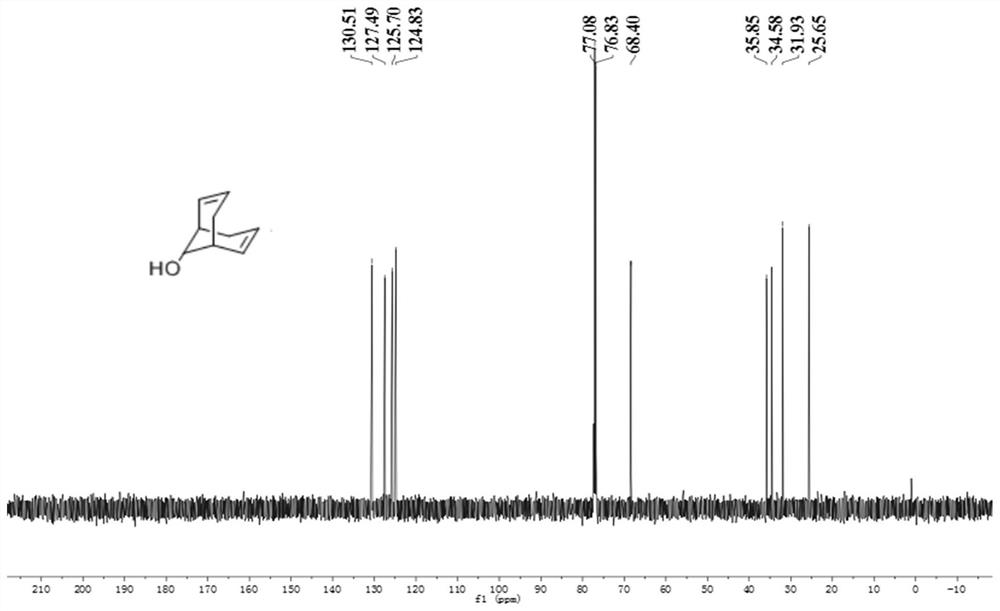
[0047] Add 5.6mL (3.50mmol) of diisopropylamine and n-butyllithium to 15mL of tetrahydrofuran successively at low temperature. After the dropwise addition is completed, add 1.008g (2.00mmol) of raw materials in batches. After the addition of raw materials is completed, slowly rise to The stirring reaction was continued at 25°C for 12h. Add 30 mL of water dropwise in an ice bath to quench the reaction, extract with ethyl acetate, adjust the pH to 4-5 with 10% dilute hydrochloric acid, wash with saturated saline, distill under reduced pressure to obtain 300 mg of golden yellow oil, and obtain 240 mg of white solid by silica gel column chromatography , yield 88%. Its characteristic graph is as figure 1 and figure 2 shown. 1 H NMR (500MHz, CDCl 3 )δ:5.92-5.51(m,4H),3.95(d,J=5.9Hz,1H),2.62-2.33(m,4H),2.07(dd,J=17.8,4.7Hz,1H),1.94-1.80 (m,2H)ppm. 13 C NMR (126MHz, CDCl 3 )δ: 130.51, 127.49, 125.70, 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com