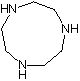
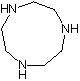
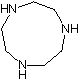
Synthesis method of 1, 4, 7-triazacyclononane
A synthetic method, nonane technology, applied in the direction of organic chemistry, can solve the problems of low total yield, low three-step molar yield, pollution, etc., and achieve the effect of avoiding side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0024] In a 500ml four-neck flask equipped with a mechanical stirrer, an air condenser, a thermometer, a dropping funnel and a dry ice acetone bath, add 25.8g of N,N'-di(2-chloroethyl)ethylenediamine hydrochloride in sequence (0.1mol), 51.6g of carbon tetrachloride. Start stirring, and cool down to minus 50°C with a dry ice acetone bath.
[0025] Slowly add 48.8g (0.4mol) of DMAP in batches. When DMAP is added into the bottle, the system will exotherm. Control the feeding speed to ensure that the temperature does not exceed minus 40°C. After adding DMAP, when the temperature dropped to minus 50°C again, start to slowly add 5.7g (0.095mol) of anhydrous ethylenediamine dropwise, and control the rate of addition to ensure that the temperature does not exceed minus 45°C. After the dropwise addition was completed, the temperature was kept at minus 50°C to minus 45°C for 3 hours. After the heat preservation was completed, the cooling bath was removed, and the reaction was stirred ...
example 2
[0029] In a 500ml four-neck flask equipped with a mechanical stirrer, an air condenser, a thermometer, a dropping funnel and a dry ice acetone bath, add 25.8g of N,N'-di(2-chloroethyl)ethylenediamine hydrochloride in sequence (0.1mol), 51.6g of carbon tetrachloride. Start stirring, and cool down to minus 50°C with a dry ice acetone bath.
[0030] Slowly add 48.8g (0.4mol) of DMAP in batches. When DMAP is added into the bottle, the system will exotherm. Control the feeding speed to ensure that the temperature does not exceed minus 40°C. After the addition of DMAP, when the temperature dropped to minus 50°C again, slowly add 6 g (0.1 mol) of anhydrous ethylenediamine dropwise, and control the rate of addition to ensure that the temperature did not exceed minus 45°C. After the dropwise addition was completed, the temperature was kept at minus 35°C to minus 30°C for 3 hours. After the heat preservation was completed, the cooling bath was removed, and the reaction was stirred at ...
example 3
[0033] In a 2000ml four-necked flask equipped with a mechanical stirrer, an air condenser, a thermometer, a dropping funnel and a dry ice acetone bath, 200 g of N,N'-bis(2-chloroethyl)ethylenediamine hydrochloride ( 0.78mol), 400g carbon tetrachloride. Start stirring, and cool down to minus 50°C with a dry ice acetone bath.
[0034] Slowly add 380g (3.1mol) DMAP in batches. When DMAP is added into the bottle, the system will exotherm. Control the feeding speed to ensure that the temperature does not exceed minus 40°C. After adding DMAP, when the temperature dropped to minus 50°C again, start to slowly add 45g (0.75mol) of anhydrous ethylenediamine dropwise, and control the rate of addition to ensure that the temperature does not exceed minus 45°C. After the dropwise addition was completed, the temperature was kept at minus 50°C to minus 45°C for 3 hours. After the heat preservation was completed, the cooling bath was removed, and the reaction was stirred at room temperature ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com